The size of my research group hovers around the 35 mark. Nowadays, it is composed, for the most part, of postdoctoral fellows and a few graduate students. Visitors at the undergraduate, graduate and faculty levels are made welcome. When it comes to the generation of new research projects, the dynamic is largely a bottom-up one. I encourage in-coming group members to introduce themselves into our midst in an incremental manner. Following short meetings with every present group member on arrival, the in-coming researcher is encouraged to join a team or two in the research laboratory. Once established, the group member is challenged to come up with his or her own ideas and assume the role of being a team-leader. Not only do I promote team-work within my group but I also look to members of my group to establish collaborations with other research groups at Northwestern University (NU). One of the major strengths of research in chemistry and materials science at NU is the fact that “we hunt in packs.”
The intellectual life of my group is sustained by weekly meetings held on Saturdays, starting at 11 am with a delivery of pizzas. Three members of the group present their most recent accomplishments in research. This meeting, which is held throughout the year, is called Group Therapy (GT) in order to differentiate it from the weekly Group Meeting (GM) which is held during term time at 5 pm on Wednesdays. GM is run like a tennis tournament, where three group members each select and speak about a recent article from the literature. After the presentations, votes are cast online to evaluate the speakers’ ability to discuss relevant background information, the paper’s contents, and a critical review. The presenter with the most votes goes forward to the next round; two semi-final rounds are followed by a final one, completing the competition.
The ambience that prevails in the research laboratory has earned the nickname of being called the Research Palace or RP for short.
When it comes to the writing up of research for publication, teams led by the first author produce drafts of a manuscript and supporting information to which I respond with written comments. Drafts can run anywhere between two or three up to more than a dozen. Before a manuscript is submitted all the authors meet in our Conference Room for a read-through. These events are highly interactive and enjoyable.
Beauty in a New Chemical Bond
Much has already been said and written about the presence of beauty in chemistry. For some chemists, beauty lies in the shape and clarity of a crystal or in the color or texture of a pigment. For others as chemists, beauty is expressed by wholly synthetic Platonic and Archimedean molecular structures, such as cubane, dodecahedrane and Buckminsterfullerene, for they can be prized for their symmetry, simplicity and uniformity. Put it another way, these structures, and many more into the bargain, are simply beautiful and beautifully simple. When, going back half a century ago, natural products, and the often referred to art of their synthesis, was all the rage, many in the chemical community were brainwashed by the great and good of the age into admiring this kind of technical wizardry for its complexity, elegance and sophistication. One can question whether it was art, or more likely, in my humble opinion, molecular engineering of exquisite beauty for those with an encyclopedic knowledge of chemical reactions.
For my own part, molecular nanotechnology has uncovered many other avenues to associate beauty with chemistry that include beautiful new ways of representing molecules using graphical representations which can be described more crudely as cartoons! My own abiding interest in molecular nanotechnology has revolved around the wonders of the mechanical bond which is ubiquitous in the macroscopic world, but has only seriously visited the molecular world during little more than the past quarter-of-a-century or so.
One of the really beautiful hallmarks of chemistry is its ability – because of its all-important making component – to keep reinventing itself over and over again. This endearing virtue places the chemist in the same arena as a painter of pictures or a sculptor of statues or a composer of music. I found myself acting out this privileged role, starting way back in the 1980s, as a maker of molecules with a brand new bond – the mechanical bond – which opened the door to crafting and producing mechanically interlocked molecules, or MIMs for short. These MIMs have arguably, not only played a central role in revolutionizing molecular nanotechnology, but they have also initiated a paradigm shift towards much more aesthetically pleasing and artistically attractive illustrations of molecules – call them graphic representations or cartoons, if you like – in the scientific literature. These eye-catching representations of MIMs are now part of the chemical lexicon, be they a molecule with two or more interlocked rings which we call a catenane, a name derived from the Latin word catena meaning chain – or a molecule, comprised of a dumbbell-shaped component, wherein a rod is threaded through one or more rings with ends (stoppers) that are too bulky for the rings to bypass – that we call a rotaxane, a name derived from the Latin words rota for a wheel and axis meaning axle.
Mechanical bonding has pervaded the natural world from time immemorial. Nature has been using mechanical bonds long before we humans came on the scene. DNA Catenanes (and knots) have constituted some of the front runners among the players in the field of biological MIMs. We are reminded constantly that Nature executes the chemistry of the mechanical bond with a level of elegance, complexity and beauty that will remain a source of inspiration to synthetic chemists for centuries to come.
Nowhere is the beauty of the mechanical bond more pervasive than in the art world. Artists have been drawing, painting and carving mechanical bonds for thousands of years. A class of MIMs that is worthy of special mention is that of the Borromean rings, a topology which consists of three rings mechanically interlocked in such a manner that the breaking of any one ring results in the whole caboodle falling apart. The mutual dependence, expressed by this topology, has rendered these rings a powerful symbol for threefold unification, originating in name from three wings of the Borromeo family in what is now known as northern Italy. It was the mechanical and physical beauty of this prevalent piece of topology present in so much art—not to mention logos—spanning many cultures for centuries, that inspired us to set out and finally make the molecular Borromean rings. After a decade of failed attempts, in 2004 we were able to synthesize the first-ever molecular Borromean rings using an approach known as dynamic covalent chemistry, which brings together no less than 18 components in one fell swoop. The dynamic nature of the synthesis also led, with a very small change in reaction conditions, to the formation of a Solomon knot (link), in which two of the three rings present in the Borromean rings form a doubly interlocked catenane. This particular topology is unconditionally handed (chiral), leading to pairs of MIMs that are related to each other as an object is to its mirror image, yet are non-superimposable and become, therefore, what chemists call a racemic mixture of enantiomers.
The mechanical bond is an integral part of our everyday lives. Rings, necklaces and belts, not to mention shirts and pants (trousers), all form mechanical bonds with our bodies and with themselves. Even before we learn how to walk and talk, we will most likely have become familiar with a vast range of mechanically interlocked toys, of which a rattle – reminiscent of a rotaxane – is one of the more common examples that undoubtedly played a part in my own design and synthesis in 1991, for the first time, of a molecular shuttle based on a degenerate rotaxane architecture. The introduction of bistability, by the breaking of symmetry, of a palindromic molecular shuttle led to molecular switches and ultimately molecular machines – all examples of MIMs based on the in-depth understanding and exploitation of the mechanical bond.
The journey from the playpen to being able to play life’s games, from understanding Nature to an appreciation of art, from learning about the microscopic world to mechanical and electrical engineering has been one of sheer joy for me. There is no denying that enjoyment is a beautiful experience, particularly when you realize that the physics which dictate the workings of micro- and nanomachines are not the physics that define and control the macroscopic world we know so well. Beauty is to be found in surprises that force us to think differently about the biological world in which we live and die. When it comes to making molecular machines, e.g., elevators, that remind us of ones we use every day then be prepared to accept that their mode of operation will be governed either by flashing energy ratchets or information ratchets. In many ways small is more beautiful than we might expect at first glance.
While there are probably many thousands of new chemical compounds that are made every week in chemical laboratories all around the world and, in any one year there may well be discovered several dozen genuinely new chemical reactions, it is only once in a blue moon that a new chemical bond, which rivals the strength of that old (covalent) chemical bond, breaks upon the scene. When something that is really new happens, it offers the opportunity to call into question the bigger picture. So it was with the advent of the mechanical bond. Not only did it open the door to the introduction of graphical representations and cartoons, it also called out loudly for the use of color to enhance the grey message that had been the use of black-and-white in chemistry for more than a century. Much to the chagrin of some in the chemical community, reds and blues, greens and purples, oranges and pinks started to invade chemistry journals starting in the late 1980s. To this day, the “little blue box” occupies a special place in chemistry, while the use of color has become commonplace throughout the chemical literature. Although, on occasions, it might come at a price, the beauty that we all associate with color has done much to brighten up chemistry, making it more accessible, more understandable and more pleasurable.
Research Key Words
Analytical Chemistry / Batteries / Biological Chemistry / Bistable Systems / Carbohydrate Chemistry / Catalysis / Catenanes / Chemical Topology / Chirality / Circular Dichroism / Coordination Chemistry / Crown Ethers / Crystallography / Cucurbiturils / Cyclodextrins / Dendrimers / Drug Delivery Systems / Dynamic Covalent Chemistry / Electrochemistry / Emergent Behavior / Flashing Energy Ratchets / Gold Chemistry / Hydrogen Bonding / Inorganic Chemistry / Information Ratchets / Isothermal Titration Calorimetry / Liquid Crystals / Macrocyclic Chemistry / Macromolecular Chemistry / Mass Spectrometry / Materials Chemistry / Mechanical Bonds / Mechanically Interlocked Molecules / Mechanostereochemistry / Mesoporous Silica Nanoparticles / Metal-Organic Frameworks / Molecular Belts / Molecular Borromean Rings / Molecular Boxes / Molecular Cages / Molecular Knots / Molecular Mechanics / Molecular Machines / Molecular Motors / Molecular Nanotechnology / Molecular Pumps / Molecular Recognition / Molecular Shuttles / Molecular Solomon Knots / Molecular Switches / Noncovalent Bonding Interactions / Nuclear Magnetic Resonance Spectroscopy / Out-of-Equilibrium Systems / Photochemistry / Physical Chemistry / Physical Organic Chemistry / Polymer Chemistry / Radical Chemistry / Rotaxanes / Second-Sphere Coordination / Self-Assembled Monolayers / Self-Assembly / Self-Replication / Stereoelectronic Effects / Stereochemistry / Structure-Directed Synthesis / Supramolecular Chemistry / Supramolecular Polymers / Template-Directed Synthesis / Synthetic Chemistry / Unnatural Product Synthesis.
RESEARCH SUMMARY
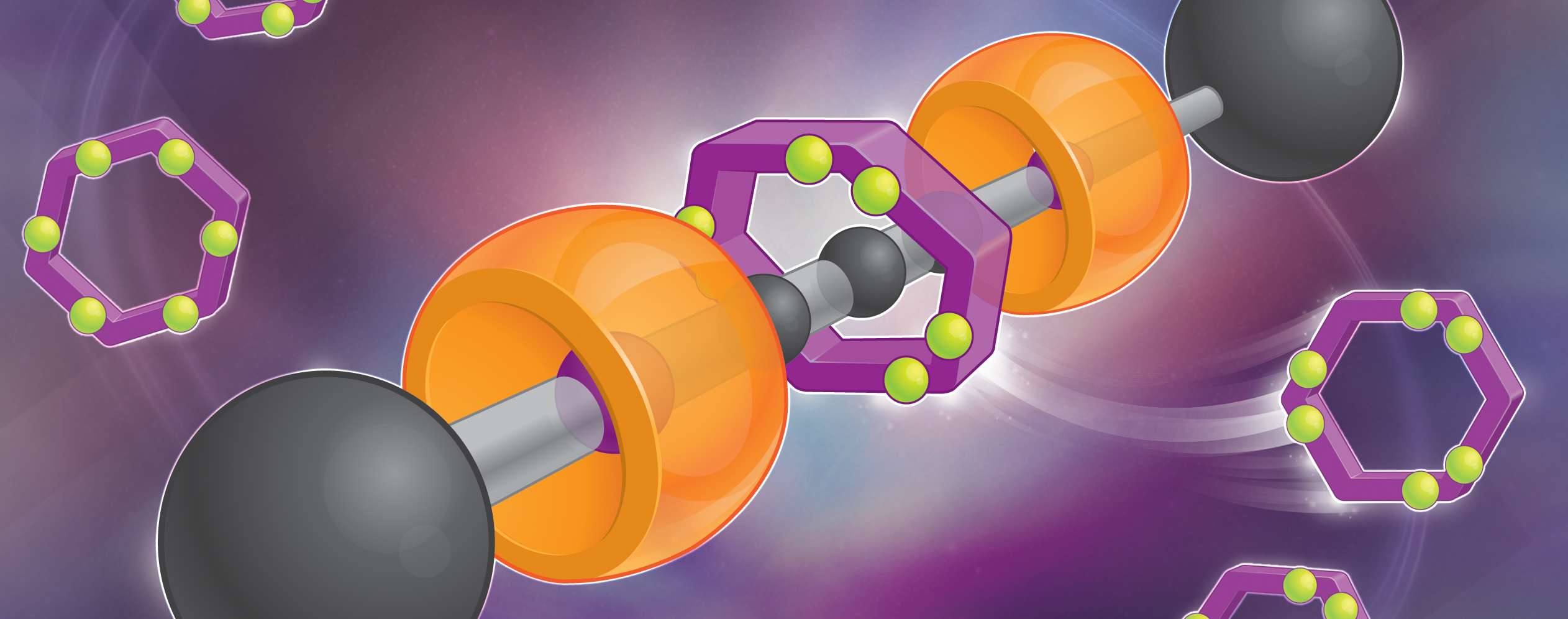
Boxes and Cages
Cyclobis(paraquat-p-phenylene) – a bipyridinium-based cyclophane – plays a central role in the development of mechanically interlocked molecules and molecular machines. A synthetic protocol, using tetrabutylammonium iodide as a catalyst, facilitates the extension of this cyclophane, resulting in a series of extended cyclophanes using a wide range of building blocks. We are exploring the emergent properties of these pyridinium-based cyclophanes, whose roles cover ground that spans (i) synthetic methodology, (ii) extraction and sequestration, (iii) physical organic chemistry, (iv) supramolecular chemistry, (v) catalysis and (vi) molecular electronics.
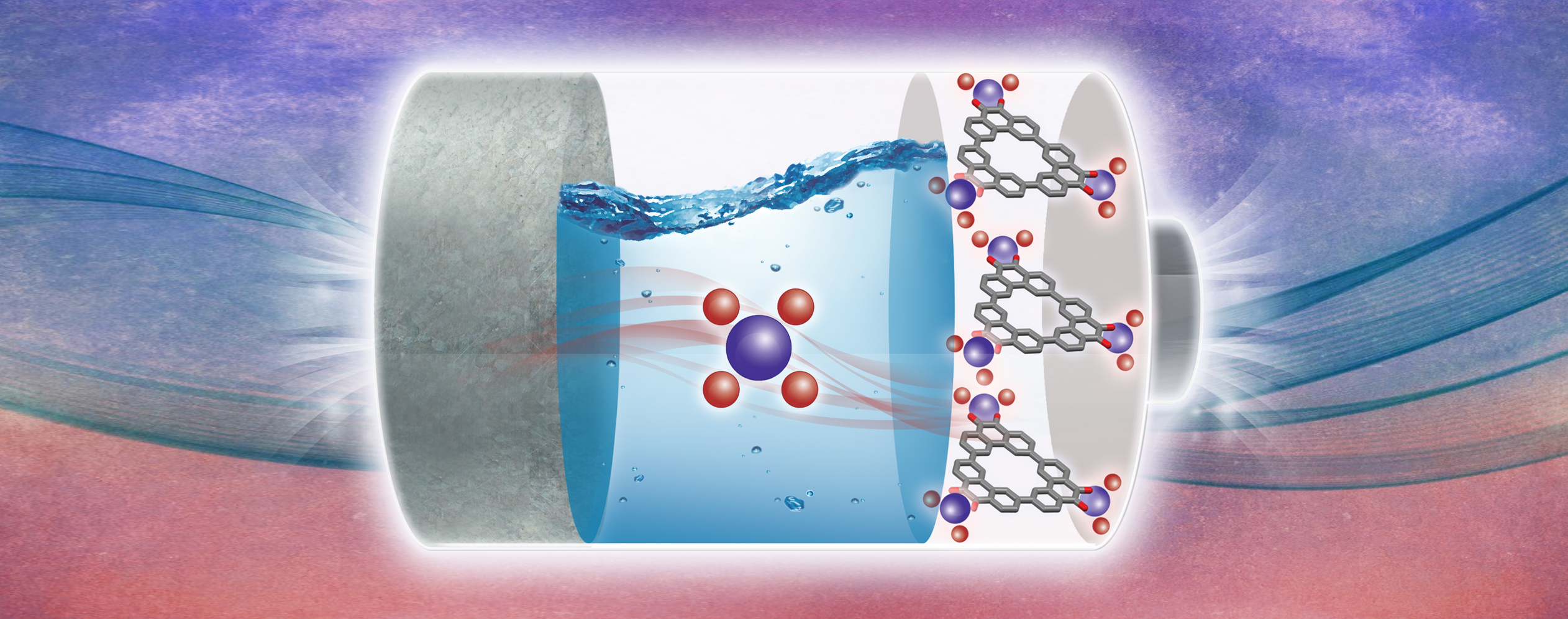
CD-Containing Frameworks
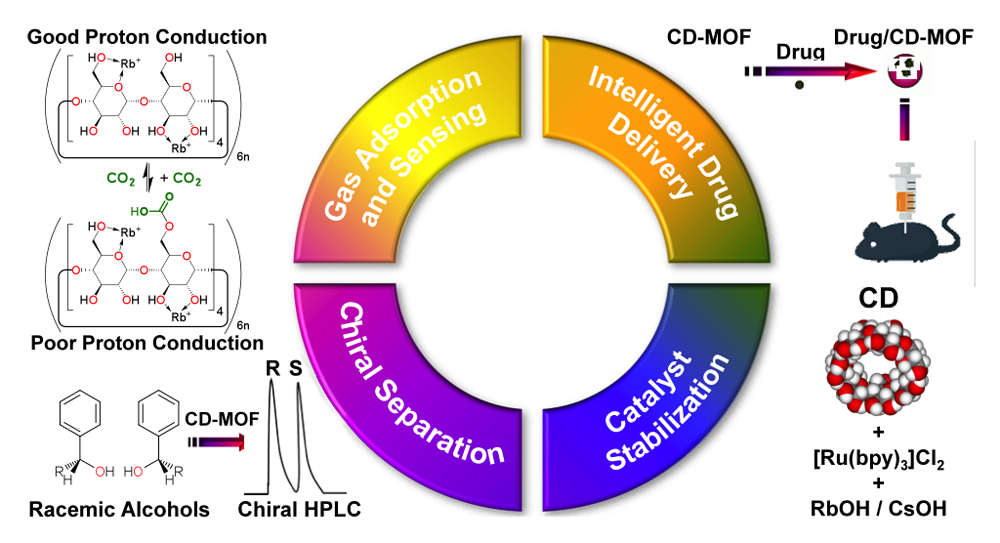
Renewable, edible, and extended porous frameworks—namely cyclodextrin-based metal‒organic frameworks (CD-MOFs) derived from γ-cyclodextrin (γ-CD) and alkali metal salts—constitute a class of metal-organic frameworks (MOFs) that can be synthesized from nontoxic, naturally occurring starting materials on a bulk scale. On account of the chiral building units present (γ-CD) present in CD-MOFs, they have the ability to exhibit molecular recognition and enantiomeric differentiation. Our research focuses on the development of new porous materials by adding to the family of CD-MOFs and increasing their moisture stability, as well as probing their versatile applications potential in areas such as gas sensing, adsorption and separation, chiral differentiation, template syntheses of metal-based nanoparticles and gels, electronic memory, optical materials, drug delivery, and catalyst stabilization.
Drug Delivery
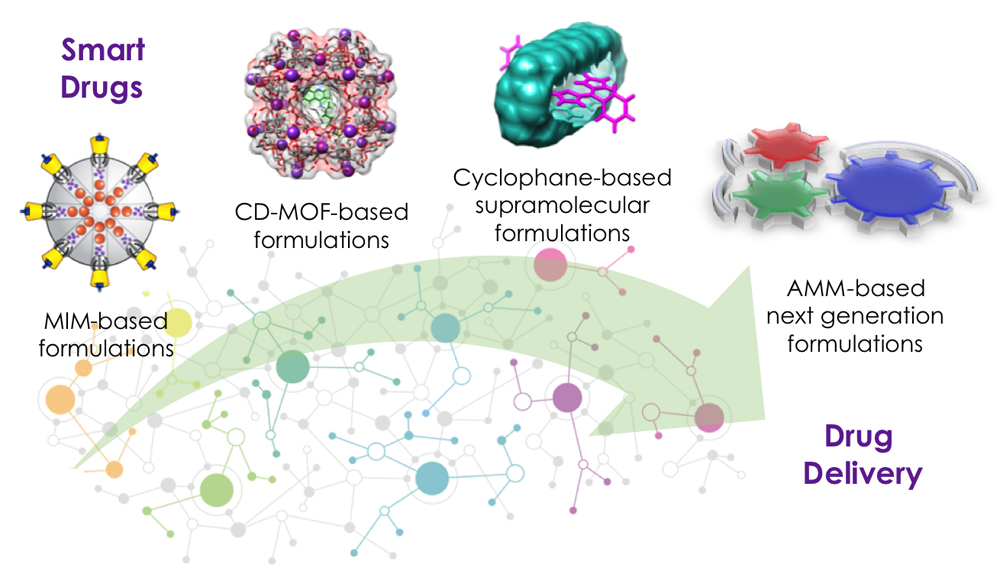
The development of new methods to administer drugs with low solubility or bioavailability, limited chemical stability, along with the need to deliver drugs selectively, and to targeted locations around the body, are amongst the most critical areas of research in drug development. Effective delivery of drugs into live-cells with spatiotemporal control is, however, challenging, on account of the fact that lots of these drug molecules cannot freely enter through the cell membrane, and they lack sustained release on reaching their target. Our research into drug delivery in the Stoddart group focuses on the exploitation of supramolecular architectures for developing smart drug formulations—employing mechanically interlocked molecules (MIMs) based porous nanoparticles, cyclodextrin metal-organic frameworks (CD-MOFs) and their composites, and cyclophanes—for targeted and programmed drug delivery into mammalian cells and in animal models. We are also working on developing next-generation drug formulations by using artificial molecular machines (AMMs).
Energy Storage
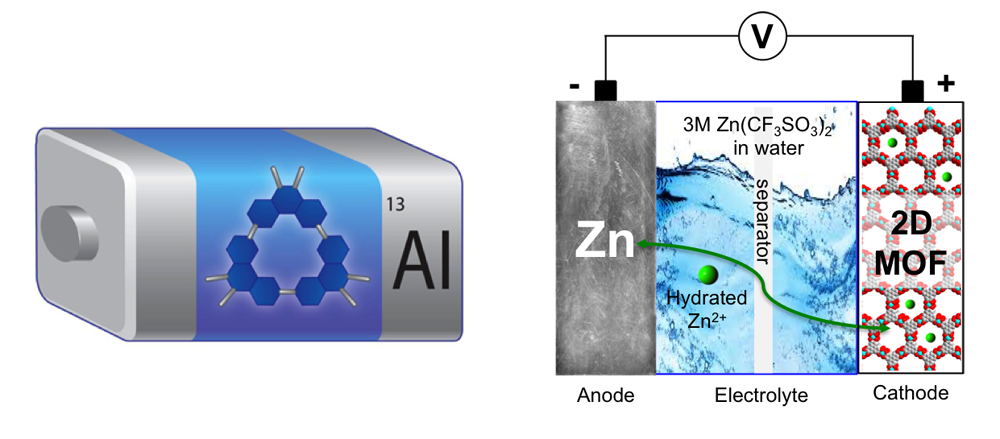
The battery community is paying increasing attention to post-lithium (Li)-ion batteries, which can transcend current Li-ion batteries with respect to key electrochemical properties, cost, and safety. As a promising alternative, rechargeable aluminum batteries (ALBs) and aqueous rechargeable zinc batteries (ZBs) are gaining considerable attention since they are cost-effective, safe and environmentally benign. ALBs and ZBs, however, still linger at the research stage, awaiting the development of high-performance cathode materials. Research on Energy Storage in the Stoddart group focuses on developing emerging materials such as redox-active macrocycles and conductive metal-organic frameworks (MOFs) in order to supply cathode materials for ALBs or ZBs.
Molecular Belts
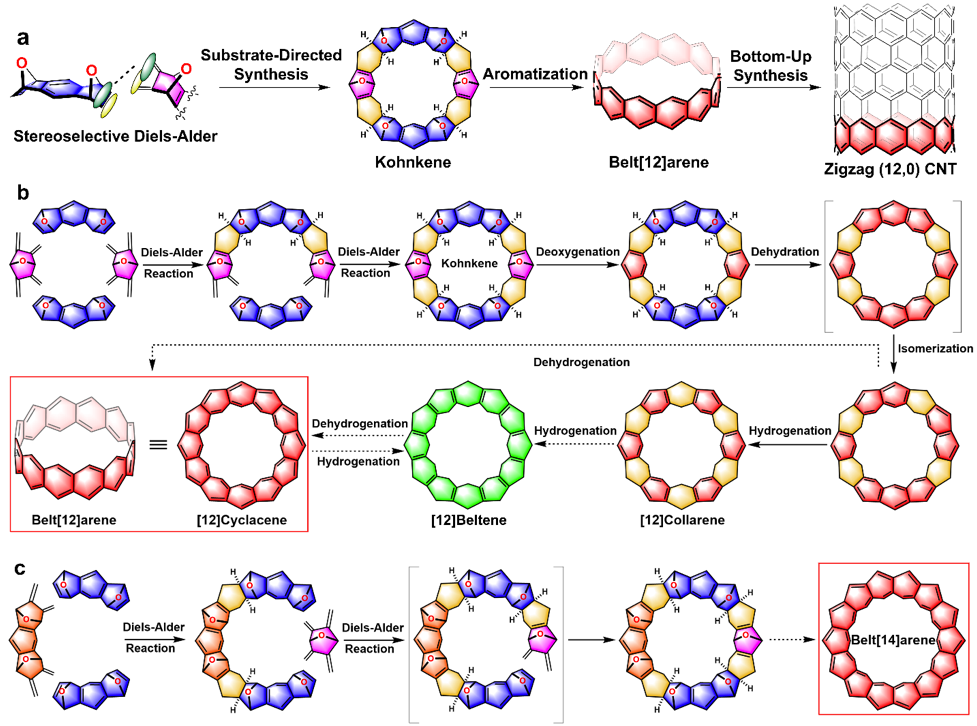
Hydrocarbon belts (HCBs) have fascinated scientists for over half a century because of their aesthetically appealing structures and potential applications in carbon nanotechnology. The foremost challenge in the synthesis of HCBs is how to build up the accumulation of energy in these highly strained structures. Successful constructions of HCBs offer, not only well-defined templates, but also practical principles that could be useful in the atomically precise and structurally predictable synthesis of uniform carbon nanotubes (CNTs) with singular chiralities—a long-standing goal in nanocarbon science.
Over three decades ago, we pioneered regio- and diastereoselective approaches to precursors of [12]cyclacene and accomplished the synthesis of [12]collarene in 1988 on the eve of the isolation of CNTs. We are currently making efforts to aromatize the partially saturated precursors into the fully fused and conjugated molecules with belt shapes—namely belt[12]arene and belt[14]arene.
Molecular Electronics
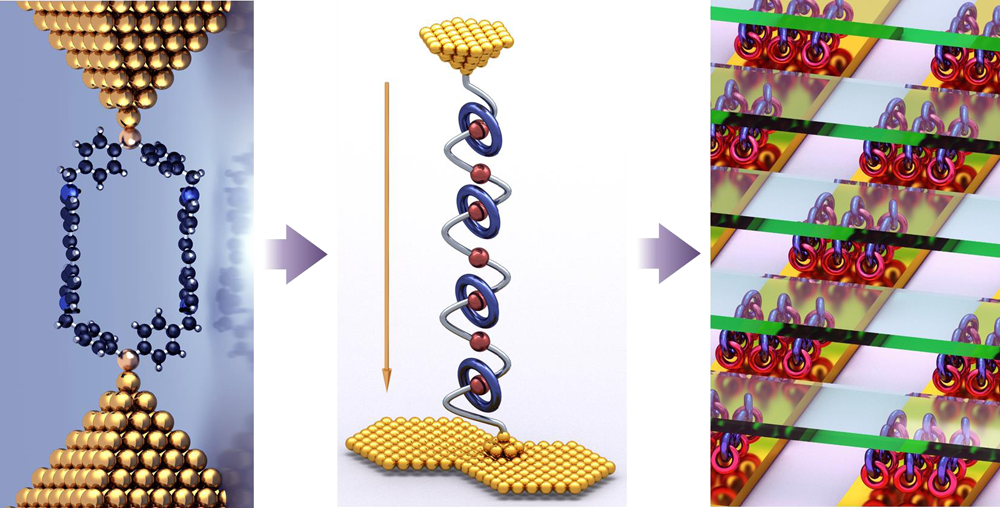
Understanding the movement of electrons to and through a single molecule is central to the field of molecular electronics which has been investigated for more than 40 years. When it comes to supermolecules or mechanically interlocked molecules (MIMs), the situation becomes more complicated. Not only is the focus on the charge transport within an individual supermolecule, but one must also pay special attention to the delicate and complex weak interactions between different components in complexes and in MIMs. Research in the Stoddart Group covers three different scales, starting with (i) investigation of the single-molecule transport properties of macrocycles and macrobicycles and their related host-guest supermolecules, (ii) followed by detecting and manipulating the weak interactions in supermolecules and MIMs, and (iii) finally applying the fundamental knowledge gained in the first two stages to construction of new single-molecule functional devices and even integrated circuits.
Molecular Machines
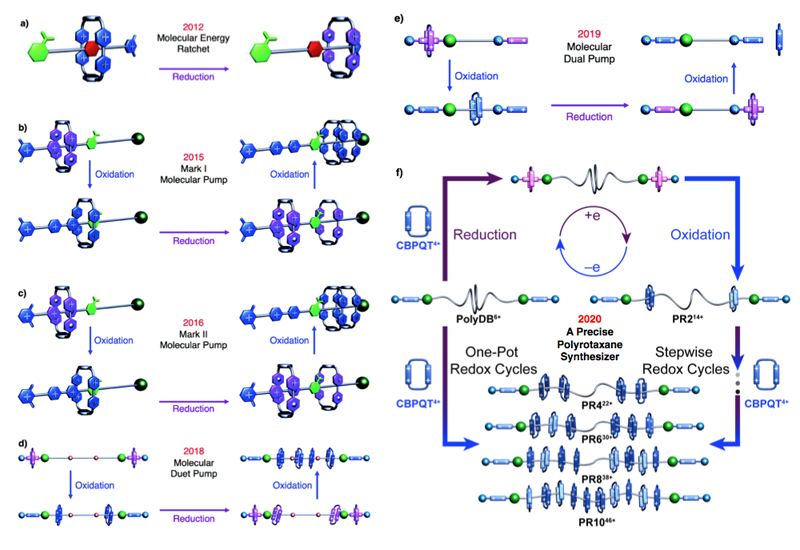
The design and synthesis of functional molecules with machine-like behavior enable chemists to pursue chemistry away-from-equilibrium. Mechanically interlocked molecules (MIMs) have been at the forefront of this research endeavor towards the production of molecular machines which can open doors to unprecedent research opportunities. We have designed and synthesized a so-called artificial molecular pump, a subset of artificial molecular machines, which is employed to produce enthalpically and entropically demanding rotaxanes and polyrotaxanes.
Molecular Photochemistry
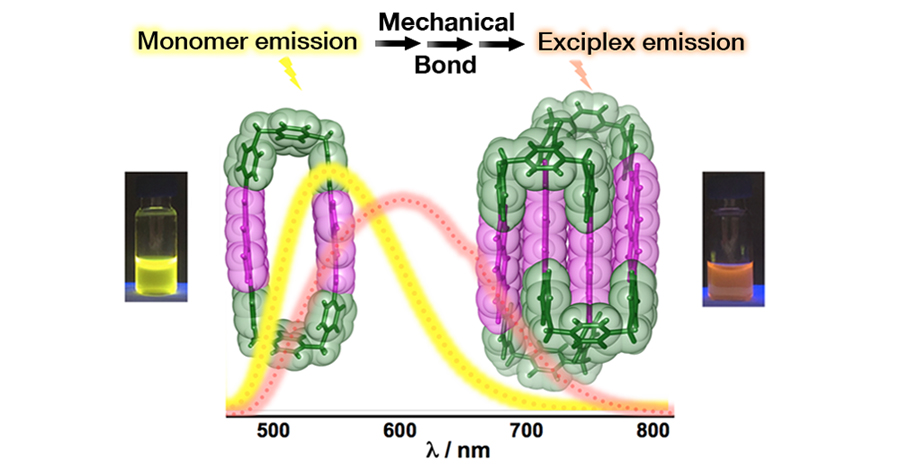
The phenomenon of photoluminescence (PL) from exciplexes presents countless opportunities for applications. Although PL has been investigated extensively, less is known about the precise control of the molecular aggregates and their persistence at very low concentrations. Our approach to obtaining permanent exciplex PL begins with the incorporation of anthracene moieties into pyridinium-containing mechanically interlocked molecules (MIMs). The difference in the optical properties of the anthracene-based cyclophanes and the precursor homo[2]catenane are consistent with the role of the mechanical bond in the emergence of the low energy exciplex emission in the catenane. Furthermore, this exciplex emission has been detected under high-dilution conditions and applied successfully in live-cell imaging. These results shed light on the importance of photoactive polyaromatic components like anthracene as self-templating systems for promoting the formation of homo[2]catenanes, while the mechanical bond can bring fluorophores into close proximity for generating permanent exciplex emissions.
Porous Materials
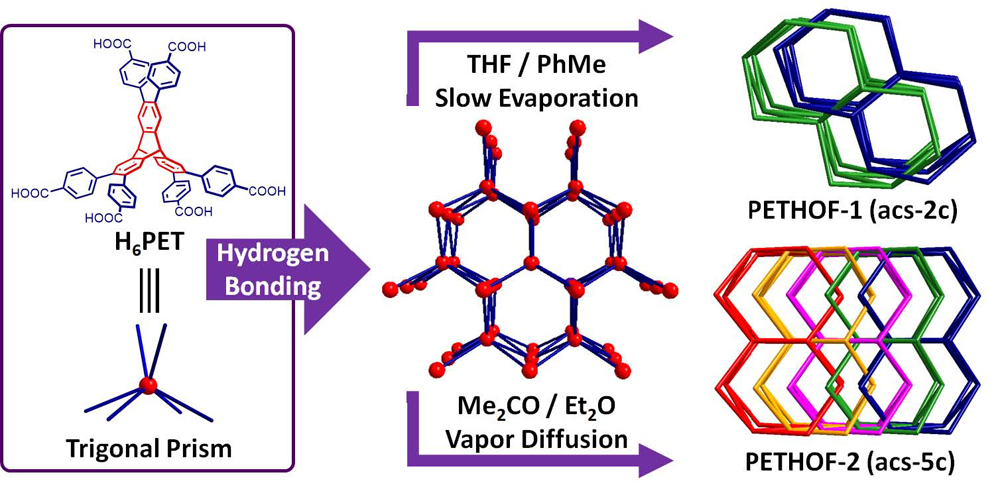
Hydrogen-bonded organic frameworks (HOFs), assembled from organic molecules by means of hydrogen bonding, are emerging as promising porous materials in conjunction with metal-organic and covalent-organic frameworks (MOFs and COFs) thanks to their ease of their synthesis under mild conditions and their solution processabilities that are generally not characteristic of robust polymeric networks. We have been exploring the use of triptycenes with peripheral aryl carboxyl groups as the building blocks to assemble 3D HOFs. These peripherally extended triptycenes (PETs), which exhibit a rigid trigonal prismatic geometry, can form porous HOFs with a variety of complex 3D supramolecular architectures. Our research in this area focuses on 1) the discovery and design of 3D HOFs with mechanically interlocked network topologies, and 2) the utilization of these porous frameworks for molecular recognition and separation.
Supramolecular Systems
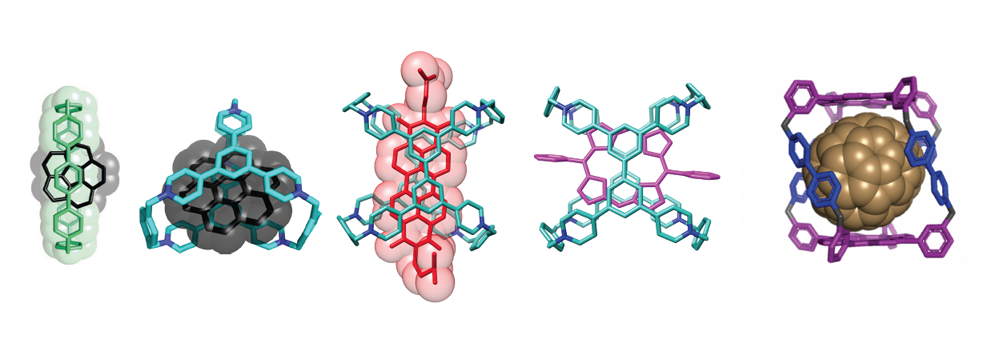 Supramolecular systems explore the properties and functions beyond molecules. Molecular recognition, a central theme of supramolecular chemistry, is the key to unlocking and releasing the hidden potential of supramolecular materials. In the Stoddart group, we design and synthesize molecular receptors with tunable cavity sizes and shapes, which selectively and effectively recognize various functional substrates and realize emergent functions in separation, catalysis, stabilization, imaging, sensing, capture and release.
Supramolecular systems explore the properties and functions beyond molecules. Molecular recognition, a central theme of supramolecular chemistry, is the key to unlocking and releasing the hidden potential of supramolecular materials. In the Stoddart group, we design and synthesize molecular receptors with tunable cavity sizes and shapes, which selectively and effectively recognize various functional substrates and realize emergent functions in separation, catalysis, stabilization, imaging, sensing, capture and release.
RESEARCH HIGHLIGHTS
Current Research
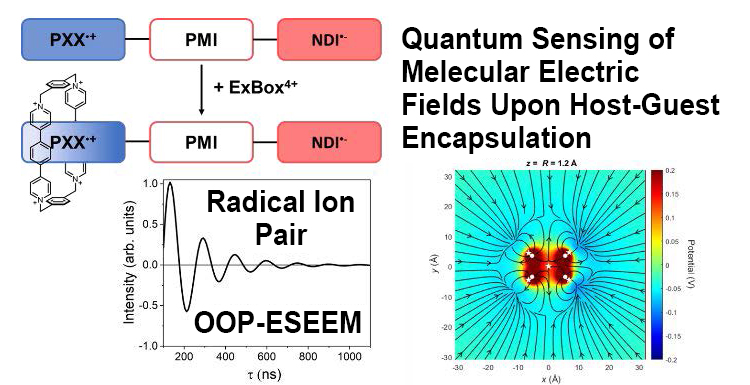
Quantum Sensing of Electric Fields Using Spin-Correlated Radical Ion Pairs / Yuanning Feng
Publications – 1284
We show that a photogenerated spin-correlated radical ion pair (SCRP) can be used to sense an electric field change created at one radical ion of the pair. We use molecular recognition to bind a charged cyclophane to one radical of the SCRP. Pulse-EPR measurements are used to measure the coherent oscillations created primarily by the electron–electron dipolar coupling in the SCRP, which yields the distance between the two charges (spins), demonstrating that the SCRP can function as a quantum sensor to detect electric field changes in the vicinity of the radical ions.

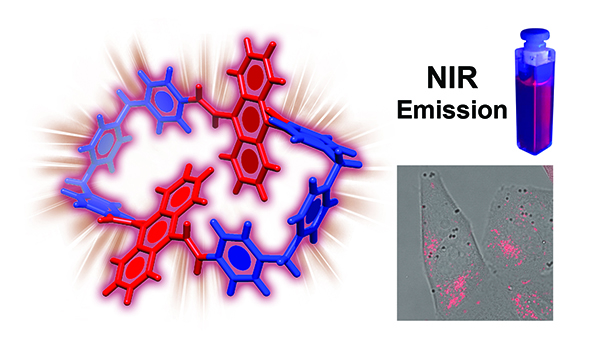
Divinylanthracene-Containing Tetracationic Organic Cyclophane with Near-Infrared Photoluminescence / Arthur David and Amine Garci
Publications – 1278
Modification of the cyclobis(paraquat-p-phenylene) cyclophane structure by insertion of two 9,10-divinylanthracene units between two of the pyridinium units led to the formation of an extended tetracationic cyclophane, namely VAnBox4+. This cyclophane emits a deep-red and near-infrared (NIR) photoluminescence. On account of its excellent water solubility, the VAnBox4+ has been applied as a NIR probe for live-cell imaging of cancerous cells.

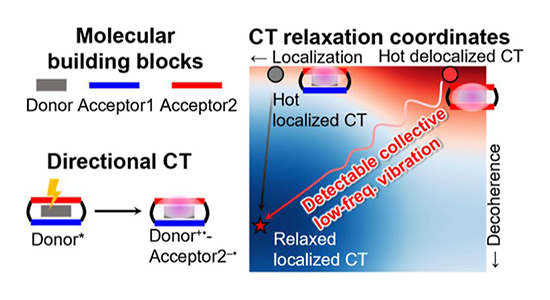
Coherent Vibronic Wavepackets Show Structure-Directed Charge Flow in Host–Guest Donor–Acceptor Complexes / Yuanning Feng
Publications – 1277
Upon photoexcitation, the asymmetric host−guest ExMeOVBox4+ ⊃ Per complex exhibits directional CT toward the energetically unfavorable methoxylated side due to structural restrictions that facilitate strong interactions between the Per donor and the ExMeOV2+ side. The CT state relaxation pathways are probed using ultrafast optical spectroscopy by focusing on coherent vibronic wavepackets, which are used to identify CT relaxations along charge localization and vibronic decoherence coordinates.vibronic wavepackets, which are used to identify CT relaxations along charge localization and vibronic decoherence coordinates. Specific low- and high-frequency nuclear motions are direct indicators of a delocalized CT state and the degree of CT character. Our results show that the CT pathway can be controlled by subtle chemical modifications of the acceptor host in addition to illustrating how coherent vibronic wavepackets can be used to probe the nature and time evolution of the CT states.

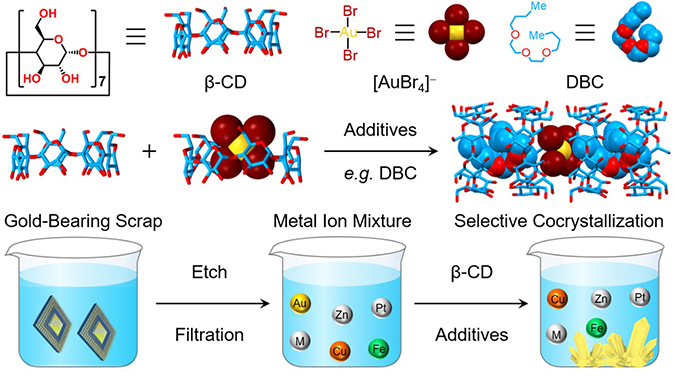
High-Efficiency Gold Recovery by Additive-Induced Supramolecular Polymerization of β-Cyclodextrin / Huang Wu
Publications – 1275
An eco-friendly supramolecular metallurgical technology for gold recovery, which is based on an additive-induced supramolecular polymerization of β-cyclodextrin and tetrabromoaurate anions, has been demonstrated. In a laboratory-scale gold-recovery protocol, over 94% of gold in electronic waste was recovered at gold concentrations as low as 9.3 ppm. This simple protocol constitutes a promising paradigm for the sustainable recovery of gold, featuring reduced energy consumption, low-cost inputs, and the avoidance of environmental pollution.

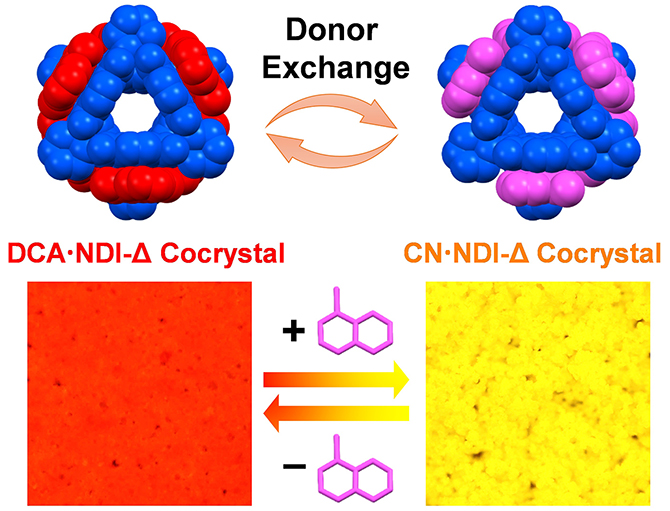
Color-Tunable Upconversion-Emission Switch Based on Cocrystal-to-Cocrystal Transformation / Yu Wang
Publications – 1273
The interconversion of two macrocycle-based cocrystals has been realized by the exchange of their electron donor molecules. Accompanying the cocrystal transformation, their two-photon excited luminescent color changes between red and yellow, forming a high-contrast dual-color upconversion-emission switch.

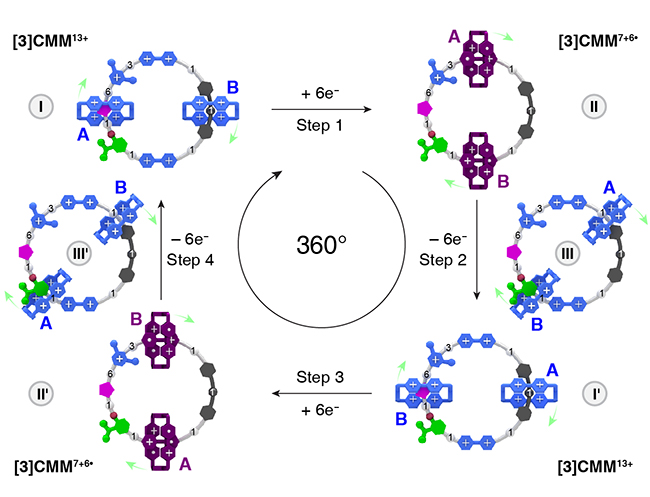
An Electric Molecular Motor / Long Zhang
Publications – 1272
Electric motors convert electrical energy into mechanical motion. A molecular motor based on a [3]catenane has been designed and synthesized in which electricity drives redox reaction cycles that lead to the movement of two small rings around a large circular loop. The small rings make one full revolution around the loop for every two redox cycles.

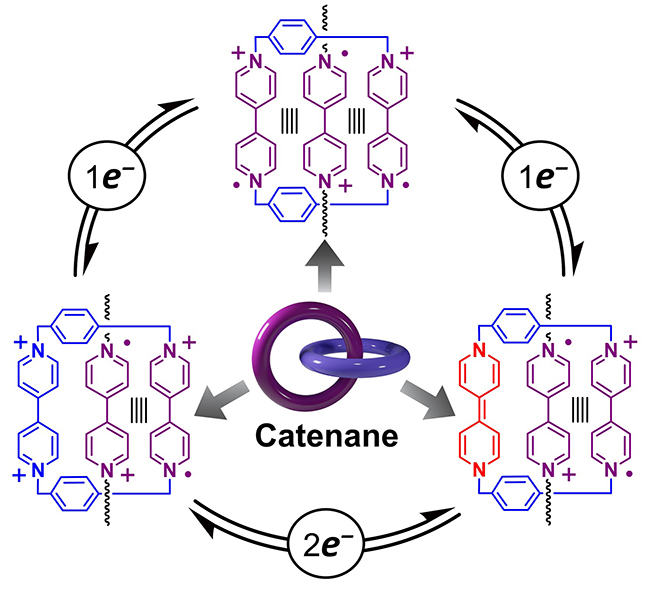
Mechanical Bond-Assisted Full-Spectrum Investigation of
Radical Interactions / Yang Jiao
Publications – 1269
This project aims to answer a fundamental scientific question of “How many kinds of complexes can be formed noncovalently between cyclobis(paraquat-p-phenylene) (CBPQT) and a 4,4′-bipyridinium (BIPY) derivative”? By employing a mechanically interlocked [2]catenane as the model compound, we have performed a full-spectrum investigation of radical interactions and revealed unambiguously a total of three possible binding modes—to be specific, a bisradical tetracationic, a trisradical tricationic and a bisradical dicationic association—as demonstrated by various methods of characterization including UV/Vis/NIR, EPR and NMR spectroscopies, electrochemical measurements and X-ray crystallographic analysis.

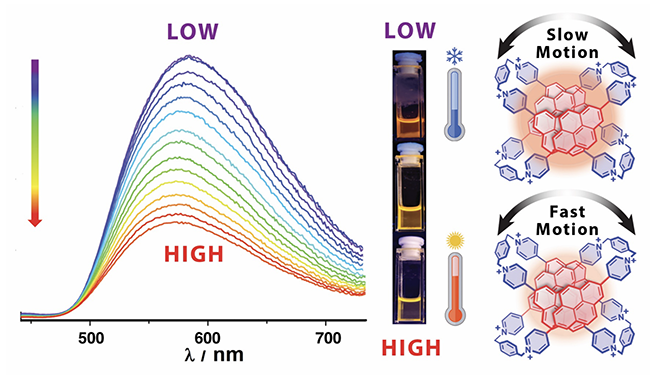
Thermally Controlled Exciplex Fluorescence in a Dynamic Homo[2]catenane / Amine Garci
Publications – 1270
This project describes the investigation of a thermoresponsive dynamic homo[2]catenane incorporating pyrene units and the relative circumrotational motions of its cyclophanes. The relative coconformational motions induce a significant change in the fluorescence emission of the homo[2]catenane upon changes in temperature compared with its component cyclophanes. This variation in the exciplex emission of the homo[2]catenane is reversible as demonstrated by four complete cooling and heating cycles. This research opens up possibilities of using the coconformational changes in MIMs-based chromophores for probing fluctuations in temperature which could lead to applications in biomedicine or materials science.

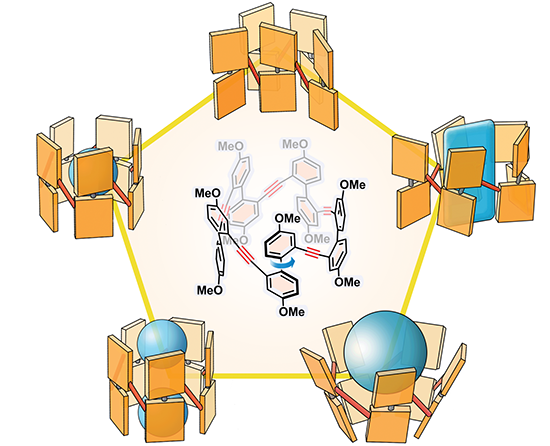
Corralarenes: A Family of Conjugated Tubular Hosts / Han Han
Publications – 1267
A new family of conjugated tubular hosts, for which we have coined the name corralarenes, have been designed and synthesized. They display bright fluorescent emission, dynamic chirality, and induced-fit guest-binding properties. The deep and adaptive cavity of corral[5]arene allows it to accommodate a wide range of guests of different molecular sizes and shapes.

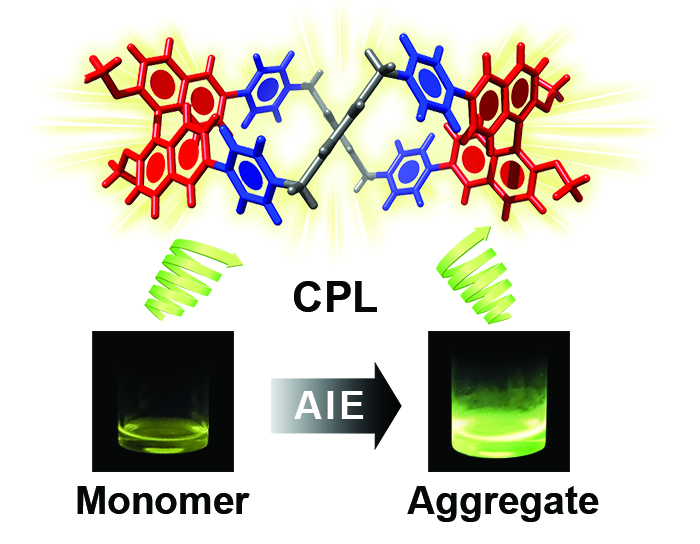
Aggregation-Induced Emission and Circularly Polarized Luminescence Duality in Tetracationic Binaphthyl-Based Cyclophanes / Amine Garci
Publications – 1261
This project describes a new approach to the synthesis of highly charged enantiopure cyclophanes by the insertion of axially chiral enantiomeric binaphthyl fluorophores into the constitutions of pyridinium-based macrocycles. Remarkably, these fluorescent tetracationic cyclophanes exhibit a significant aggregation induced emission compared to their neutral optically active binaphthyl precursors. Furthermore, these highly charged enantiopure cyclophanes display circularly polarized luminescence responses both in solution and in the aggregated state. This unique duality of aggregation induced emission and circularly polarized luminescence in these tetracationic cyclophanes is destined to be of major importance in future development of photonic devices and bio-applications.

Precise non-equilibrium polypropylene glycol polyrotaxanes / James Seale
Publications – 1260
Molecular pumps were used for the synthesis of a series of non-equilibrium polyrotaxanes of cyclobis(paraquat-p-phenylene) rings threaded on polypropylene glycol polymer chains. The pumping of just two rings was sufficient to render these hydrophobic polymers soluble in aqueous media, and the gradual degradation of the polyrotaxane—a manifestation of their non-equilibrium nature—was characterized.

Quadrupolar Fluorescent Dyes / Yuanning Feng
Publications – 1259
We have designed and synthesized a series of fluorescent dyes that contain [acceptor-donor-acceptor] quadrupolar backbones. Their quadrupolarity-correlated photophysical properties includes a range of fluorescent colors which cover the visible spectrum from deep violet to green. Steady-state and transient absorption and emission spectroscopic analysis have allowed us to gain deeper insight into intramolecular charge transfer. A non-cytotoxic water-soluble dye performs in vitro live-cell confocal microscopic images that show cell uptake at relatively low concentrations.

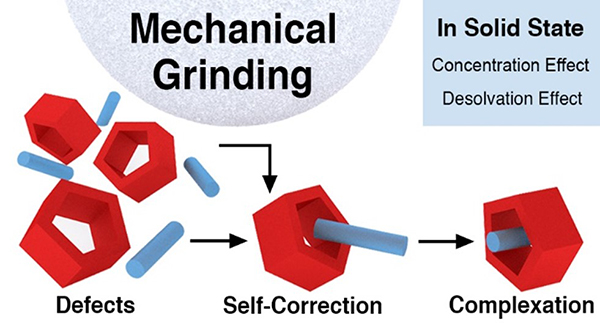
Mechanochemical Enhancement of the Structural Stability of Pseudorotaxane Intermediates in the Synthesis of Rotaxanes / Tae-woo Kwon
Publications – 1257
Pseudorotaxane defects—that is, free host and guest molecules—turns out to be self-corrected upon mechanical grinding. This remarkable finding allowed us to synthesize rotaxanes based on host-guest pairs having extremely low binding constants in high yields. Mechanochemistry holds promise as a potential tool in synthesis of mechanically interlocked molecules that are nigh impossible to prepare in solution on account of their weak intercomponent interactions.

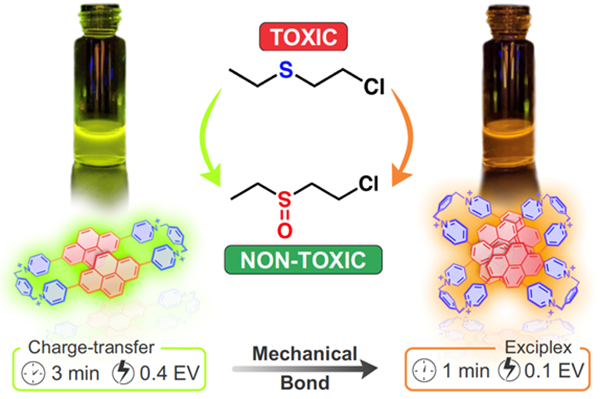
Mechanically Interlocked Pyrene-Based Photocatalysts / Amine Garci
Publications – 1254
This project describes a new approach to fine-tune the photophysical properties of pyrene—a well-known organic photosensitizer. This strategy consists of inserting pyrene into the skeleton of mechanically interlocked molecules (MIMs). These MIMs which consist of tetra-chromophoric pyridinium-based homo[2]catenane showed significant triplet populations which led to high photocatalytic activities as demonstrated by the oxidation of a sulfur mustard simulant. This approach is tunable. Therefore, a variety of organic photosensitizers are under investigations, depending on their abilities to form homo[2]catenanes with high photocatalytic activities.
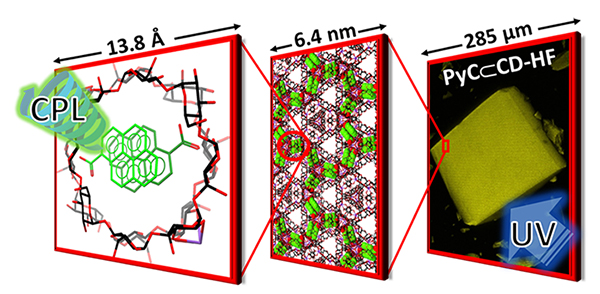
Helically Chiral Hybrid Cyclodextrin Metal-Organic Framework Exhibiting Circularly Polarized Luminescence / Masoud Kazem-Rostami
Publications – 1253
The incorporation of two achiral pyrene molecules inside the tunnel of cyclodextrin metal-organic framework lead to a helix-like position of π-stacked pyrene dimers. These dimers possess an additional axial chirality resulting in an emission of circularly polarized light.

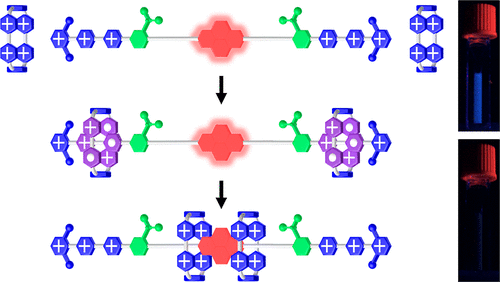
Fluorescence Quenching by Redox Molecular Pumping / Xuesong Li and Arthur H.G. David
Publications – 1247
The quenching of fluorescence of an emissive pyrene-containing dumbbell-like molecule has been achieved using the molecular pumping of CBPQT4+ rings. This quenching does not happen when these two components, i.e., the dumbbell and CBPQT4+, are mixed together without the formation of mechanical bonds.

Radically Enhanced Dual Recognition / Xiao-Yang Chen
Publications – 1242
Selective recognition of two stacked viologen radical cations in solution has been achieved using a size-matched radical host. The resulting tetrakisradical tetracationic recognition motif heralds much promise for the design of new mechanically interlocked molecules and artificial molecular machines.

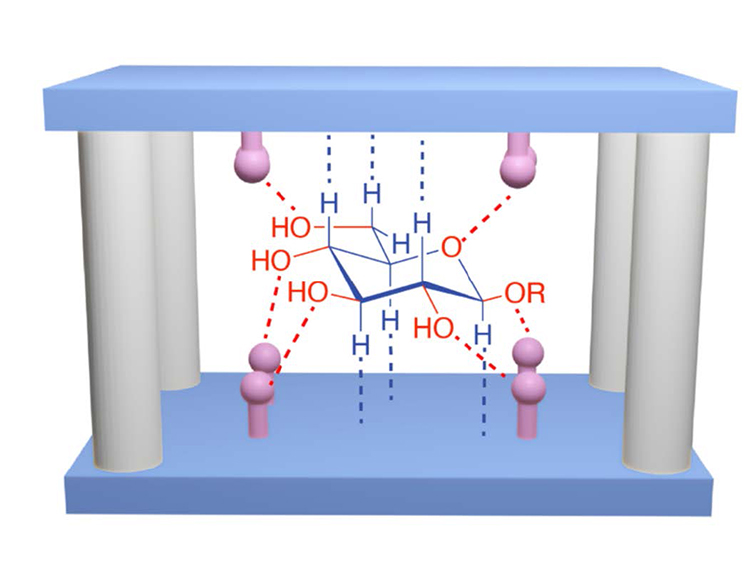
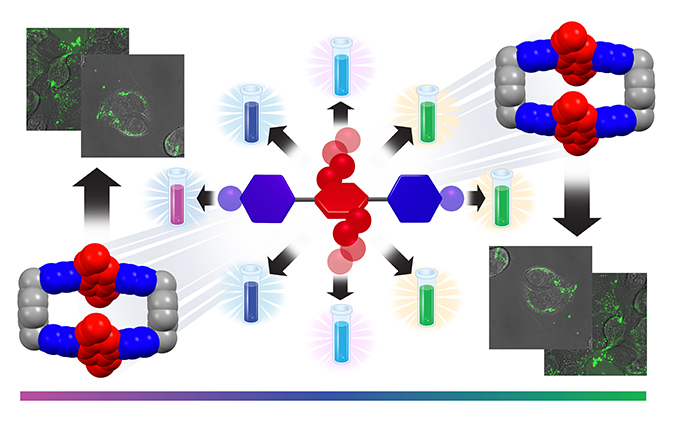
Binding Sugars in Water Inside PCages / Wenqi (Vince) Liu
Publications – 1238
Pyrene-based molecular cages, known as PCages, recognize selectively and effectively glucose in water. These receptors adopt a temple shape, wherein its stereoelectronic distribution matches that of glucose, which induces an enhancement of fluorescence in the receptors upon binding. These PCages could be applied to the design of a glucose-sensing device for the diagnosis of diabetes. This discovery suggests that the pyridinium-based molecular cages could be applied for the molecular recognition of highly hydrophilic substrates in aqueous media.

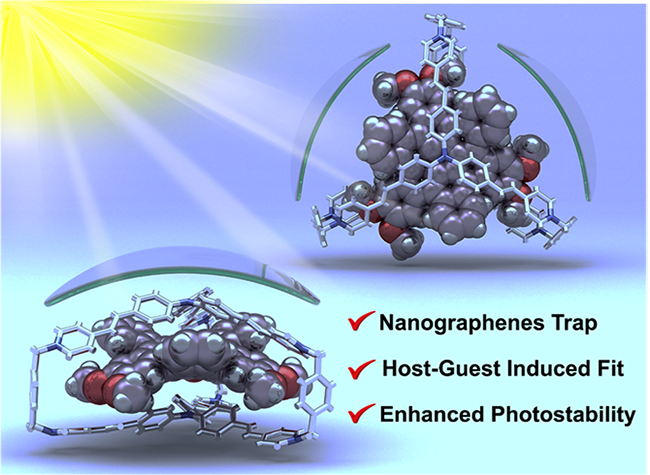
A Contorted Nanographene Shelter / Huang Wu
Publications – 1235
A trigonal prismatic hexacationic cage, TPACage6+, serves as a receptor not only for planar coronene, but also for contorted nanographene derivatives. The photostability of the nanographenes is improved significantly by the ultrafast deactivation of their excited states within the cage.

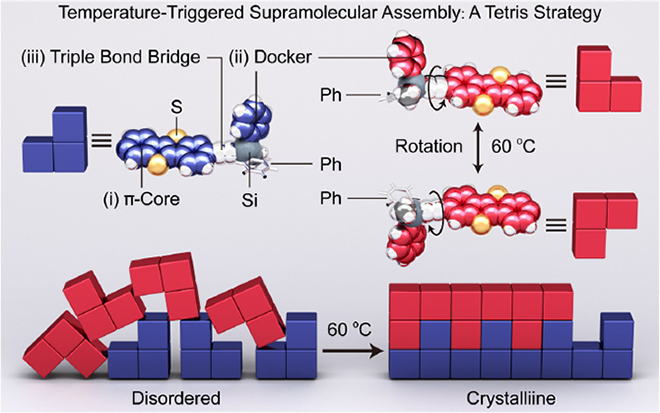
Temperature-Triggered Supramolecular Assembly of Organic Semiconductors / Hongliang Chen
Publications – 1234
A temperature-triggered supramolecular assembly strategy is used for fabricating highly ordered organic semiconductor arrays. The central concept of molecular design — named the Tetris strategy — is to increase the rotational freedom of the molecules through thermal perturbation, thereby inducing conformational locking of adjacent molecules as a result of cooperative [π•••π] interactions.

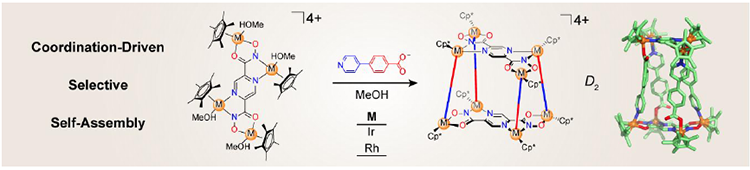
Coordination-Driven Selective Formation of D2 Symmetric Octanuclear Organometallic Cages / Long Zhang
Publications – 1233
Selective syntheses of D2 symmetric octanuclear organometallic cages from asymmetric ambidentate pyridyl-carboxylate ligands and half-sandwich iridium- and rhodium-based building blocks have been achieved by utilizing the electronic effects from two types of chelating sites (O,O′) and (N,N′). The strategy presented, not only enriches the toolbox for the construction of discrete supramolecular coordination complexes, but also reveals the fundamental understanding of the influence of electronic effects on coordination-driven self-assembly.

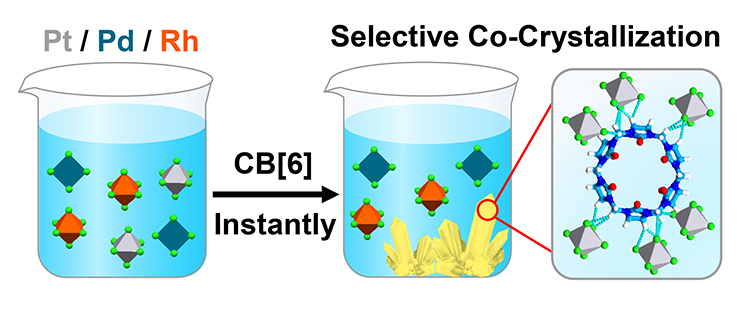
Platinum Separation and Recovery Based on Exo-Binding with Cucurbit[6]uril / Huang Wu
Publications – 1232
An instantaneous co-crystallization protocol relying on the selective recognition of the [PtCl6]2– dianions by the exo-binding of cucurbit[6]uril has been demonstrated. This protocol could be exploited to recover platinum from spent vehicular three-way catalytic converters and other platinum-bearing metal scrap in a fast, efficient and highly selective manner.

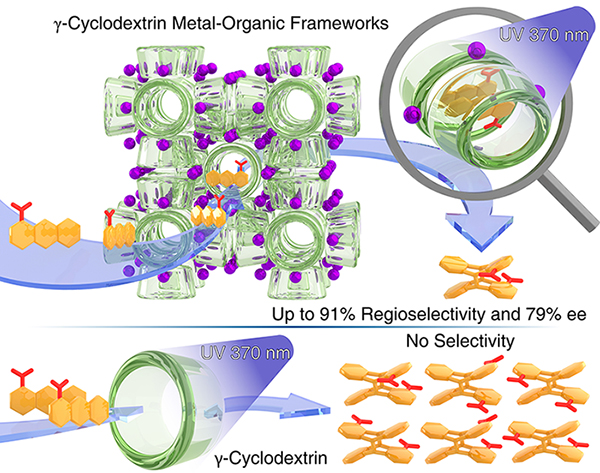
Selective Photodimerizaton in a Cyclodextrin Metal–Organic Framework / Xiaoyang Chen
Publications – 1228
Cyclodextrin-Containing Metal Organic Frameworks (CD-MOFs) serve as robust nanoreactors for the regio- and stereoselective photodimerization of 1-anthracenecarboxylate. The role of the (γ-CD)2 tunnels in CD-MOFs resembles that of the active sites in enzymes with the exception that enzymes usually have only one active site, whereas a small CD-MOF crystal—e.g., in the micrometer size range—contains billions of active sites.

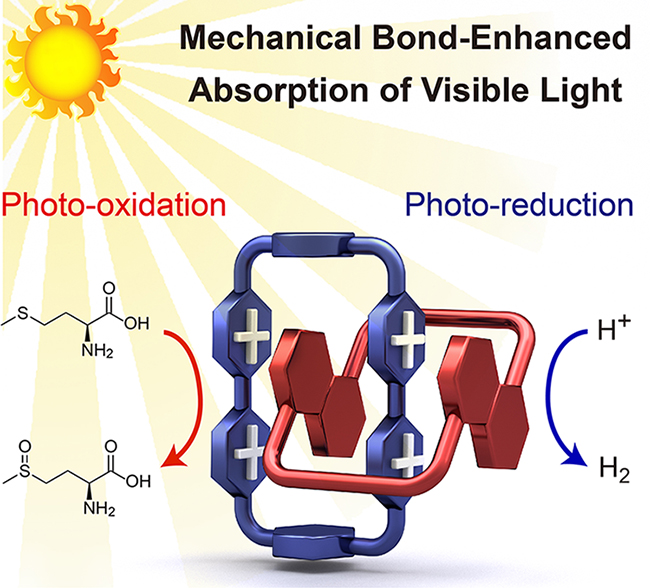
A Donor–Acceptor [2]Catenane for Visible Light Photocatalysis / Yang Jiao
Publications – 1225
A donor–acceptor [2]catenane, composed by an electron-deficient cyclophane and an electron-rich crown ether, has been employed for visible-light photocatalysis in water without the need for additional photosensitizers. As a result of mechanical bond-enhanced charge-transfer interactions, this [2]catenane harnesses visible light efficiently, promoting both photocatalytic hydrogen production and selective photo-oxidation of organic sulfides.

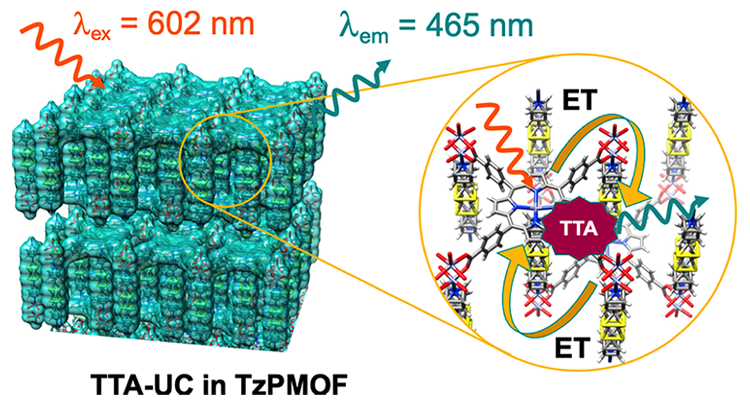
Photon Upconversion in a Glowing Metal-Organic Framework / Indranil Roy
Publications – 1220
A glowing MOF constructed from a porphyrin-based sensitizer and thiazolothiazole-based annihilator can perform efficient photon upconversion. The precise arrangements of sensitizers with respect to annihilators, and the high annihilator-to-sensitizer ratio, facilitate Dexter energy transfer, leading to high TTA-UC efficiency at low excitation power density.

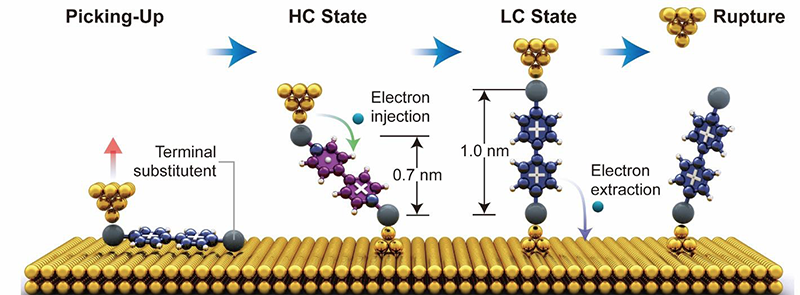
Electrostatic Anchors for Molecular Electronics / Hongliang Chen
Publications – 1217
Positively charged pyridinium units can serve as efficient electrostatic anchors to form robust gold–molecule–gold junctions. Binary conductance switching in such a dicationic viologen molecular junctions has been observed by dint of an electron-injection-induced redox-switching mechanism.

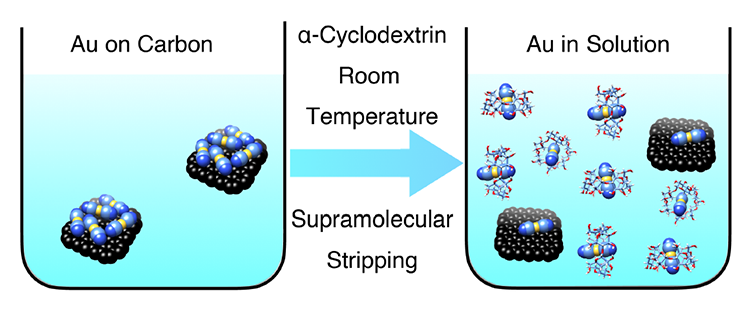
Supramolecular Gold Stripping From Activated Carbons Using α-Cyclodextrin / Wenqi (Vince) Liu
Publications – 1216
A molecular receptor for potassium dicyanoaurate, a crucial intermediate in today’s gold mining industry, turns out to be α-cyclodextrin. This remarkable act of molecular recognition has been applied to the selective stripping of gold from the surface of activated carbon into aqueous solution at room temperature.

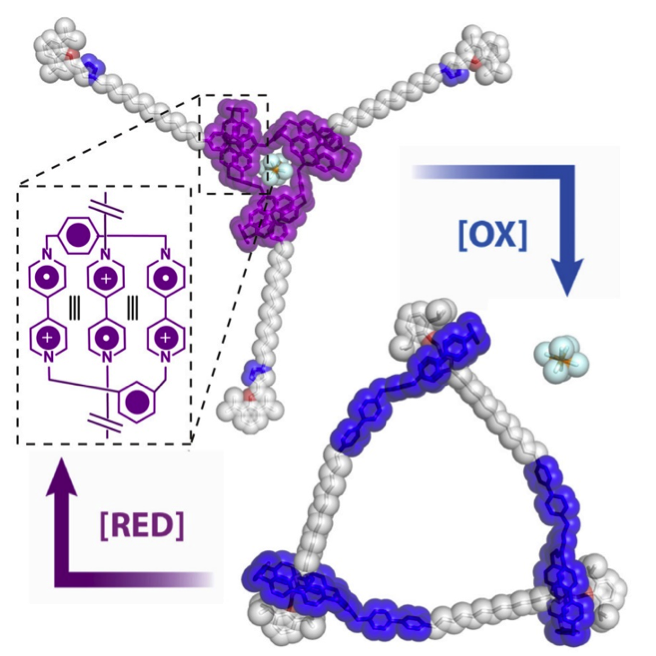
Radical Cyclic [3]Daisy Chains / Yunyan Qiu
Publications – 1214
The design and synthesis of two cyclic [3]daisy chains ([c3]DCs), namely, [c3]DC2·18PF6 and [c3]DC12·18PF6, are demonstrated using a combination of radical and anion templation. Two mechanically interlocked [c3]DCs with 18 positive charges were obtained in >90% yields. One [c3]DC displayed good air stability in its radical cationic form, while the other underwent reversible “co-conformational” switching between open macrocyclic and closed trisarm-shaped forms under electrochemical control.

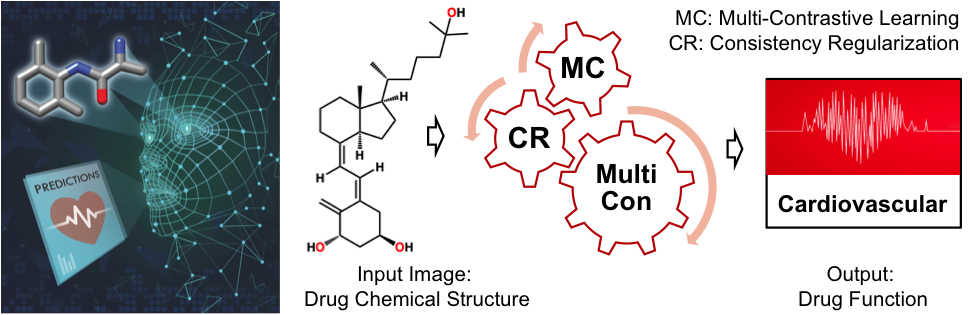
Semi-Supervised Learning for Predicting Drug Function / Indranil Roy
Publications – 1212
A new multicomponent semi-supervised learning algorithm—MultiCon—has been developed for classifying drugs into 12 categories, according to therapeutic applications, based on image analyses of their structural formulas. MultiCon achieves better prediction accuracies when compared with the other state-of-the-art machine learning methods across a variety of existing semi-supervised learning benchmarks.

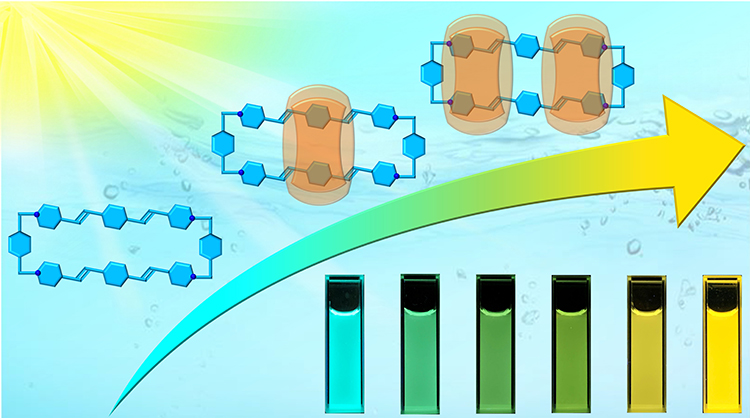
Ring-in-Ring(s) Complexes Exhibiting Tunable Multicolor Photoluminescence / Huang Wu
Publications – 1207
Binary and ternary ring-in-ring(s) complexes, which were designed and synthesized based on an extended tetracationic cyclophane and cucurbit[8]uril, exhibit tunable multicolor-fluorescence outputs.

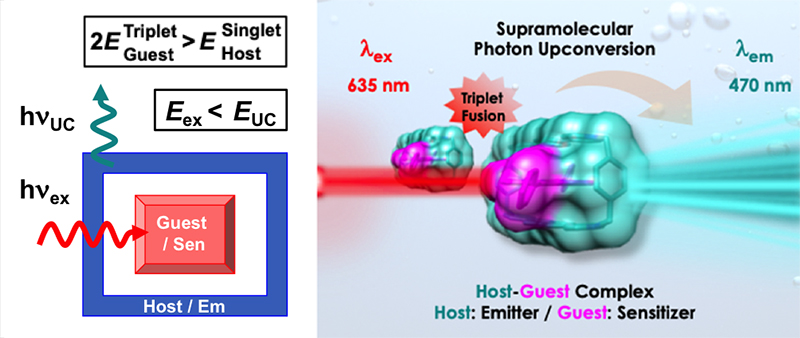
Supramolecular Photon Upconversion / Indranil Roy
Publications – 1206
A host-guest complexation mediated strategy for harnessing triplet-fusion photon upconversion is demonstrated. When a low energy red light excites the guest in a host-guest inclusion complex, ExTzBox4+⊃DPP, a high energy blue light is emitted from the host. Two times of the guest triplet energy needs to be higher than the singlet state energy of the host for this photophysical event to occur.

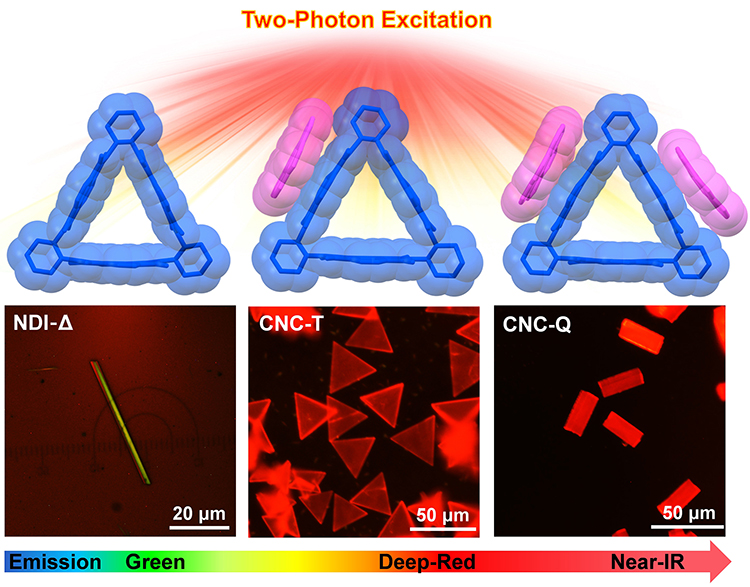
Two-Photon Deep-Red Near-Infrared Emissive Co-Crystals / Yu Wang
Publications – 1205
A convenient and efficient supramolecular approach is developed to design two-photon excite deep-red and NIR emissive materials based on the co-crystalization of a naphthalenediimide-based triangular macrocycle and coronene.

Ongoing Research
Seifallah Abid / Postdoctoral Fellow
Project 1 – Synthesis of redox switchable dendrimers
Project 2 – Porphyrin-based boxes and catenanes for oxygen sensitization
Project 3 – Perylene- and pyrene-based catenanes – with Amine Garci

Xiao-Yang (Aspen) Chen / Postdoctoral Fellow
Project 1 – Suit[3]ane
Project 2 – Photodimerization in CD-MOF
Project 3 – Radically enhanced dual recognition

Partha Jyoti Das / Postdoctoral Fellow
Project 1 – Hexacationic fluorescent molecular cage for live-cell imaging
Project 2 – Extended porphyrin cage for selective catalysis for hydroformylation Reactions
Project 3 – Second-generation hexacationic molecular cage for suitane synthesis

Arthur David / Postdoctoral Fellow
Project 1 – Fluorescence quenching through molecular pumping – with Xuesong Li
Project 2 – A nanographene-based tetracationic macrocycle or a [2]catenane
Project 3 – Switchable cation recognition in a macrocycle through radical-pairing interactions

Yuanning Feng / Postdoctoral Fellow
Project 1 – Design and synthesis a fluorescent cyclophane
Project 2 – A recoverable photo-control molecular pump
Project 3 – A cage in a cage

Liang Feng / Postdoctoral Fellow
Project 1 – Incorporating linear and rotary motors into metal-organic frameworks
Project 2 – Adding to physisorption and chemisorption, the phenomenon of away-from-equilibrium mechanisorption
Project 3 – Designing and synthesizing molecular cells and factories

Amine Garci / Postdoctoral Fellow
Project 1 – Mechanically interlocked photocatalysts: Design, synthesis and photocatalytic properties
Project 2 – Energy transfer in mechanically interlocked molecules: Design, synthesis and photophysical properties
Project 3 – Chiral luminescence in mechanically interlocked molecules: Design, synthesis and chiral properties

Yang Jiao / Postdoctoral Fellow
Project 1 – Electron-catalyzed molecular recognition
Project 2 – Full-spectrum investigation of radical-pairing interactions using a [2]catenane model compound

Masoud Kazem-Rostami / Postdoctoral Fellow
Project 1 – Chiroptical and switchable mechanically interlocked systems
Project 2 – Densely functionalized ligands for applied MOFs
Project 3 – Photoswitchable cyclophanes – with former member Alan Enciso

Tae-woo Kwon / Postdoctoral Fellow
Project 1 – Mechanochemical synthesis of rotaxanes
Project 2 – Designing and synthesizing heteroatom-embedded carbon nanobelts
Project 3 – Designing and synthesizing pillarene-based rotaxanes

Penghao Li / Postdoctoral Fellow
Project 1 – Geometrically flexible naonocarbons and their cocrystals with fullerenes
Project 2 – Triptycene-based porous hydrogen-bonded organic frameworks

Xuesong Li / Postdoctoral Fellow
Project 1 – Applications of molecular pumps
Project 2 – Molecular energy ratchet
Project 3 – Radical [c3]daisy-chain-based molecular switch, polymers and materials

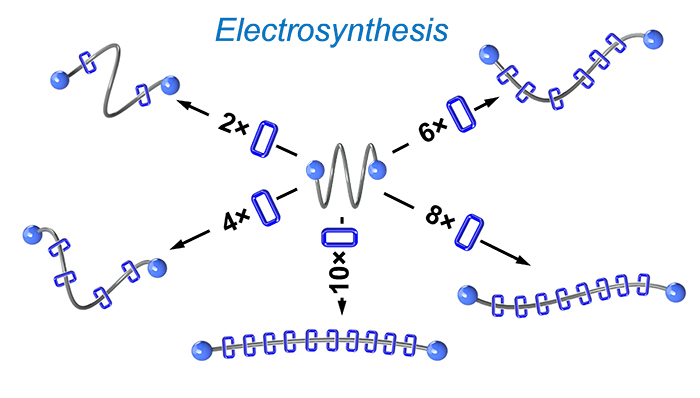
Yunyan Qiu / Postdoctoral Fellow
Project 1 – An automated electrochemical polyrotaxane synthesizer
Project 2 – Degradable polyrotaxane synthesis by artificial molecular pumps
Project 3 – A sequence-controlled polyrotaxane synthesizer

Indranil Roy / Postdoctoral Fellow
Project 1 – Molecular machines for cargo delivery
Project 2 – Incorporating linear and rotary motors into MOFs and COFs
Project 3 – Sequence-controlled polymer synthesis

James Seale / Graduate Student
Project 1 – Integrating our molecular pump into solid organic materials
Project 2 – Synthesizing a micellar system displaying artificial molecular pumps on its surface
Project 3 – Investigating how structural modifications affect a molecular pump’s energy landscape

Bo Song / Postdoctoral Fellow
Project 1 – Synthesis of molecular irises
Project 2 – Synthesis of large molecular daisy chains with 6-, 7-, and 8-membered rings
Project 3 – Construction of poly[n]rotaxanes using coordination-driven self-assembly

Yu Wang / Postdoctoral Fellow
Project 1 – Two-photon excited near-infrared emissive organic cocrystals
Project 2 – Organic charge transfer cocrystals for optoelectronic applications
Project 3 – Self-assembly of halogen-bonded organic frameworks

Huang Wu / Postdoctoral Fellow
Project 1 – A contorted nanographene shelter
Project 2 – Electronic communication within a ring-in-rings complex
Project 3 – Developing next-generation gold recovery technology using second-sphere coordination

Yong Wu / Postdoctoral Fellow
Project 1 – Precise synthesis of carbohydrate nanotubes (CNTs)
Project 2 – Wholly synthetic cyclic oligosaccharide-based MOFs

Long Zhang / Postdoctoral Fellow
Project 1 – An electrochemical molecular motor
Project 2 – Highly efficient syntheses of polyrotaxanes through radical-pairing interactions
Project 3 – Synergistic effect of Coulombic repulsion and steric hindrance in the design of artificial molecular pumps

Xingang Zhao / Postdoctoral Fellow
Project 1 – Stabilizing radical ions within a perylene diimide-cage
Project 2 – Application of a tetracationic NDI-Box
Project 3 – Hetero radical interaction for host-guest recognition

RESEARCH ENVIRONMENT
Research Facilities
The main office suite is located in Ryan Hall and is divided into three offices of similar sizes, one for Dr Stoddart, one for Dr Margaret (Peggy) Schott, Dr Stoddart’s Personal Assistant, and one for use by visiting professors. Two new offices have been commissioned in the past year and house Stephanie Teterycz (Director of Operations) and Dr Mark Olson (Associate Research Professor).
The laboratory space devoted to wet chemistry is housed in Silverman Hall 2710 and comprises ca. 600 m2 of floor space equipped with 33 energy-efficient ultra-low flow fume hoods. Each fume hood is adjacent to personal work benches, while communal bench space accommodates laboratory equipment used routinely. Three interconnected rooms of about 50 m2 host a supplies delivery and check-in area, a flammable solvent storage cupboard, consumable supplies shelving, and a chemical waste collection area, in what is colloquially referred to as the “Boom Room”. Off of the main laboratory space, three instrumentation rooms (about 90 m2 of total space) house a selection of larger instruments and equipment, as well as providing additional storage space, and two extra fume hoods which are located in these rooms and are used for sample preparation. Across the hallway from the main laboratory space, a separate room (40 m2) houses the main chemical inventory (4000+ chemicals). Office space for postdoctoral fellows and graduate students is all along one side of the laboratory in the form of rooms that house 2–7 researchers. The laboratory also has an integral and dedicated conference room (18 seats) and a small cloakroom. Adjacent to the research laboratory is a shared meeting room. Both the conference room and meeting room are outfitted with A/V equipment. All this space has been designed and built to a remarkably high standard, so much so that it is referred to by the ca. 35 group members as the RP—which is short for the Research Palace!
Research Equipment
The research laboratory is well equipped with all the tackle one would wish for in a modern laboratory where making, measuring and modelling are carried out. Each fume hood is equipped with a minimum of two hot-plate stirrers, heating mantles, a vacuum pump, and a Schlenk-line for synthetic manipulations in inert atmosphere. In order to satisfy the workload of the laboratory, we run 11 rotary evaporators—each one with its dedicated vacuum pump and pressure controller—two centrifuges for sample separation, and 10 laboratory balances for weighing materials as low as 0.01 mg. Dry solvents for syntheses are produced in-house using a solvent purification/drying system (DMF/CH2Cl2/THF/MeCN/PhMe). We produce our own ultra-pure water (18 MOhm). In addition, we are equipped with three temperature-controlled ovens for sample preparation and drying, a spin-coater for thin-film deposition, an ozone-cleaner for surface activation, and three sonicators for cleaning, sample dispersion and dissolution.
The following pieces of equipment are available —
Argon Glove-Box
Equipment details: Pure Lab HE Two Glove-Box
How is this equipment helping our research?
- Assemble lithium-ion batteries based on organic electrolytes
- Perform reactions that are sensitive to moisture and oxygen
- Grow single crystals of organic radicals that are very sensitive to oxygen and moisture.
Atomic Force / Scanning Tunneling Microscope
Equipment details: Asylum Research MFP-3D Origin / In-house built STM break junction
How is this equipment helping our research?
- Surface imaging (x-y plane) on films, surfaces, and in liquid samples using AFM techniques
- Superior force measurements (z-axis) on single molecules, complexes, and nanometer-scale structures
- Acoustic isolation for vibration suppression
- Glove-box enclosure for measurement in inert atmosphere (N2)
- Electrochemical and liquid sample holders allow for the measurement of samples submerged in liquids and upon application of an electrochemical potential.
- STM enables us to explore the electronic properties of individual molecules by ‘wiring’ them up into nanoscale junctions.
Battery Analyzer
Equipment details: MTI Corp. 8-channel battery analyzer
How is this equipment helping our research?
With this equipment we can evaluate the performance of batteries made in-house with a standard 2032 coin-cell, including charge-discharge cycles, capacity, and cycle life.
Electrochemistry
Equipment details: Gamry 600 Potentiostat
How is this equipment helping our research?
- Measure oxidation and reduction potentials for electro-active molecules, including the number of electrons exchanged and the reversibility of the redox process, in aqueous and non-aqueous solvents
- Determine binding constants for supramolecular systems, also in combination with spectrophotometric studies—spectroelectrochemistry
- Determine electrochemical kinetic constants in supramolecular systems or mechanically interlocked molecules.
- Perform bulk electrolysis using dedicated electrolytical cells with frit-, membrane- or a chamber-separated half-cell—preparative electrochemistry
Flash Chromatography
Equipment details: Teledyne ISCO Combiflash Rf 200 (normal phase) / Combiflash Nextgen 300+ (reverse phase)
How is this equipment helping our research?
- Isocratic and gradient elution purification of reaction mixtures in medium-pressure flash chromatography conditions (0-120 bar)
- Normal phase for low-medium polarity mixtures—Combiflash Rf 200
- Reverse phase for polar and charged compounds (e.g., bipyridinium derivatives) on C18-functionalized silica—Combiflash Nextgen 300+
- UV-Vis dual channel detection and automatic fraction collection
- Screening of small-scale reactions (<50 mg) or purification of preparative scale reactions (1 g)
Fluorometer
Equipment details: Horiba Fluoromax-4 Spectrofluorometer
How is this equipment helping our research?
- Collect excitation and emission spectra on solutions, powders or films of emissive materials
- Measure absolute quantum yields—using an integrating sphere
- Determine excited state lifetimes—range 200 ps – 0.1 ms
Isothermal Titration Calorimeter
Equipment details: TA Instruments – Affinity ITC
How is this equipment helping our research?
Isothermal Titration Calorimetry is a physical technique used to determine the parameters of a binding event in solution. We can determine constants ranging from 102 M–1 and up to 1012 M–1.
- Thermodynamic parameters, such as: free energy, enthalpy, and entropy of a chemical process
- Association constant of a binding event
- Stoichiometry of a complex
LC-MS (Analytical)
Equipment details: Agilent Infinity LC 1260 Quad-pump / Single Quadrupole MS 6120
How is this equipment helping our research?
- Reverse phase isocratic/gradient elution of analytical samples of small polar molecules
- UV-Vis and MS detection
- Fully automated with autosampler or manual injection
Microwave Reactor
Equipment details: Biotage Microwave Initiator 2.5 – with automatic sample loader
How is this equipment helping our research?
- Screen reactions faster and on smaller scale in a fully automated mode – up to 8 samples in queue
- Achieve temperatures and pressures not attainable easily through conventional heating
- Superior control of reaction temperature and time
- Reactions are typically complete in one-tenth of the time it would take through conventional heating
Nitrogen Glove Box
Equipment details: Innovative Technology
How is this equipment helping our research?
This glove box assists us in performing long-term reactions in the absence of oxygen. For example: reductions and reactions of radicals of bipyridinium species. Additionally, it is used for the slow crystallization of radical species into single crystals suitable for XRD structure determination.
Optical Microscope
Equipment details: Olympus BX3 Polarized Light Microscope
How is this equipment helping our research?
- Inspection of crystalline materials, single-crystals, films, and particles in solution
- Polarized light for characterization of single-crystals, liquid-crystalline films
- Trans- and epi-illumination, possibility of imaging of fluorescent species with UV-excitation
- Image collection with a cooled CCD camera
Spectrophotometers
Equipment details: Shimadzu UV-Vis-NIR 3600 / 3600Plus
How is this equipment helping our research?
- Collect the absorption spectrum of molecules in solution or solid state in the UV, visible, and near infra-red regions of the electromagnetic spectrum (190 – 3300 nm)
- Measure spectrophotometric association and kinetic constants
The 3600 Model is equipped with a variable temperature stage for measurements between 0 °C and 100 °C.
Safety in the Research Laboratory
Safety in the laboratory is at the top of the Stoddart group’s and Northwestern’s priorities. We strive to provide one of the cleanest and safest chemistry laboratory spaces on the campus by constantly keeping our day-to-day work practices up-to-date with the current safety standards and through the practice of having all of our students, postdoctoral researchers, and managers trained in safe practices and hazard mitigation.
The Office for Research Safety (ORS) (link: https://researchsafety.northwestern.edu/) surveys all safety operations on campus and administers periodical training modules through the LUMEN online platform (link: https://lumen.northwestern.edu/). Biannual safety inspections conduced by the campus safety officer are organized to identify all potential safety issues and correct them promptly. In addition, the group’s managers and safety designate perform regular inspections to highlight minor safety issues, while regular, quarterly clean-ups help us by keeping the laboratory and office spaces tidy and organized.
Researchers are equipped with state-of-the-art personal protective equipment (PPE); safety equipment, such as full showers, eye showers, first-aid kits, and spill kits are disseminated throughout the laboratory space. All our researchers receive hands-on training on the use of fire extinguishers and first intervention against small fires.
With the safety of our researchers our first priority, we have implemented a milestone-based transition plan to improve constantly the safety culture and reduce chemical hazards, not only internally, but with the intent of communicate our successful strategies to ORS and across campus.
- Reducing the generation of chemical waste (link: https://researchsafety.northwestern.edu/hazardous-waste/hazardous-waste-disposal-guide/).
- Transition to waterless condensers (link: https://www.asynt.com/products/condensyn-air-condensers/)
- Transition from oil heating baths to modular heating blocks (link: https://www.asynt.com/products/drysyn-range/)
- Lab-specific standard operating procedures (SOPs) for hazardous techniques or reagents (in progress)
- Reduction of hazardous chemical inventory (in progress).
Living in Evanston and Chicagoland
Nestled on the southwestern shore of Lake Michigan, almost 10 million residents call home the third metropolitan area by size in the US. “Chicagoland” offers a vibrant cultural environment all-year around.
Living nearby Northwestern’s campus in Evanston will grant you fast access to the university and many services. Affordable housing options—in the range of $1000-$1400 / month for a one-bedroom apartment—are available within walking distance to the building (Silverman Hall) housing the Stoddart laboratory. Rents drop as one goes further west or south of downtown Evanston. Another hot spot for living is in the northernmost neighborhood of Chicago, Rogers Park. More specifically, accommodation can be found along the tracks near Red Line stations at Loyola, Morse, and Jarvis, giving you access to a fast commute to and from Northwestern’s campus.
Evanston is well served by an efficient public transportation system. Our own “EL” train is the Purple Line, which connects the city of Evanston to the Chicago Loop with express trains during rush hours. Chicago Transit Authority Buses and Metra Commuter trains complete a capillary network of transportation that will allow you to reach virtually anywhere in the greater Chicago area.
By living in Evanston or Rogers Park you will have ready access to a variety of shopping and dining opportunities, in one of the most diverse and most well-educated communities in the country. Plenty of out-of-doors recreational activities are possible during the warmer months—May through September—and continue indoors during the harsher winter months. Sports and music events, art festivals, cultural fairs, and numerous green areas and beaches on the Lake are accessible year-long and will make your experience a unique one.
Living in a densely populated area can be stressful for some. Evanston and Rogers Park are safe communities; use common sense and avoid walking alone or in poorly frequented areas at night.
USEFUL LINKS:
Transportation:
Northwestern Shuttle Bus https://www.northwestern.edu/transportation-parking/shuttles/routes/
CTA https://www.transitchicago.com/
Metra https://metrarail.com/
Airports – O’Hare / Midway https://www.flychicago.com/Pages/default.aspx
Train – Amtrak https://www.amtrak.com/home
Driver’s License / Car Registration:
IL Secretary of State https://www.cyberdriveillinois.com/services/motorist.html
Schools:
Evanston District 65 (K-8) https://www.district65.net/
Evanston Township High-School https://www.eths.k12.il.us/
Chicago Public School System (K-12) https://cps.edu/Pages/home.aspx
Events:
Chicago https://www.chicago.gov/city/en/depts/dca.html
Things to do in the Chicago area
https://www.choosechicago.com/