A research article entitled “Alkoxy-Substituted Quadrupolar Fluorescent Dyes” has been published recently in Journal of the American Chemical Society by Postdoctoral Fellow Yuanning Feng.
Polar and polarizable π-conjugated organic molecules containing push–pull chromophores attracted a lot of attention of late. The design and synthesis of fluorescent dyes containing push–pull chromophores has emerged as a hot area of research over the past two decades as a result of their potential for applications in the fields of photovoltaics, non-linear optics, organic light-emitting diodes (OLED), fluorescent sensors, and bioimaging.
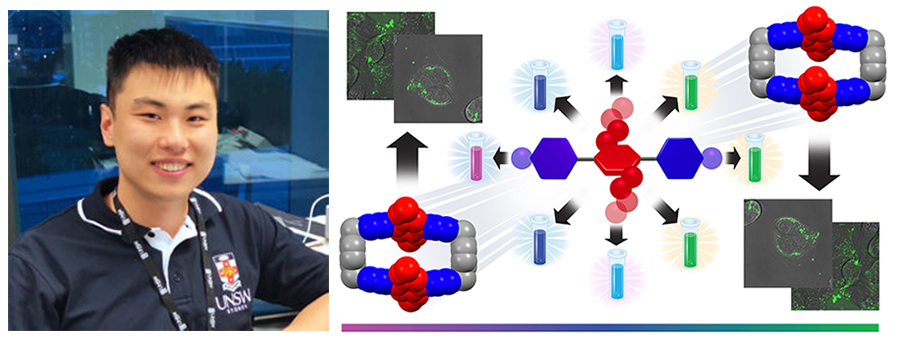
In the research described in this full paper, a rational approach to the design and synthesis of a series of fluorescent dyes that contain either [acceptor-donor-acceptor] quadrupolar para-terphenylene or extended-bipyridine/bipyridinium backbones is presented. The dyes have been functionalized with electron-rich donor rings in their midriffs and terminal acceptor rings of varying electron deficiencies, as dictated by the strength of the electron withdrawing groups (EWGs) — namely, F / CF3 / CN / COOMe / NO2 — or pyridine / substituted pyridinium units. Their quadrupolarity-correlated photophysical properties includes a range of fluorescent colors which cover the visible spectrum from deep violet to green. Steady-state and transient absorption and emission spectroscopic analysis have been performed — with a collaboration with the Wasielewski group — to gain deeper insight into intramolecular charge transfer. This investigation also demonstrates the non-cytotoxicity profile of one of the water-soluble dyes and obtain in vitro live-cell confocal microscopic images that show cell uptake at relatively low concentrations.
Pictured below – Yuanning Feng alongside the TOC for his JACS publication “Alkoxy-Substituted Quadrupolar Fluorescent Dyes”: