A research article published in Nature—by Postdoctoral Fellow Yang Jiao and Research Assistant Professor Yunyan Qiu in collaboration with our in-house Spectroscopist Michael Wasielewski, Computational Chemist William Goddard from California Institute of Technology and Theoretical Physicist Dean Astumian from University of Maine—entitled “Electron-Catalysed Molecular Recognition” has been highlighted in the latest issue of Chem.
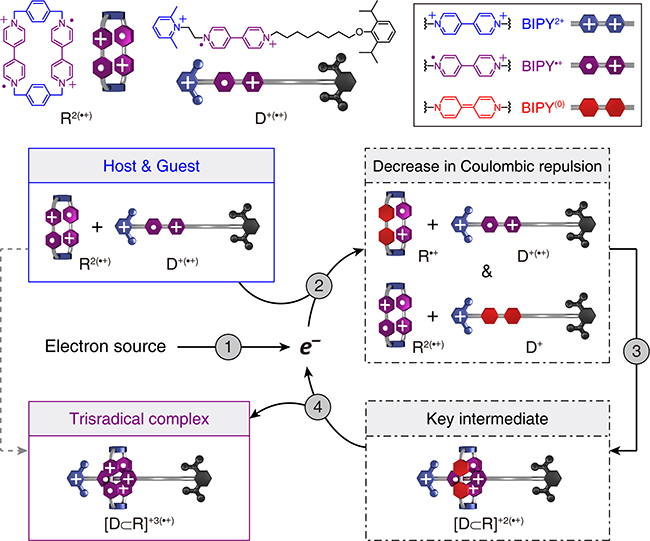
Our research article demonstrates the use of the electron, an elementary particle, to promote and control molecular recognition in both chemical and electrochemical settings. By extending the electron catalysis—a widely applied concept in synthetic covalent chemistry—into the realm of supramolecular noncovalent chemistry, the research team has established a simple, efficient and versatile strategy for catalytic assembly. Since the principle of electron catalysis circumvents the elaborate structural design of catalysts, this strategy can be readily extended to other noncovalent events or even covalent / noncovalent hybrid processes. Additionally, electrochemically controlled molecular recognition is also achieved by electrolysis in an undivided cell. This protocol, featuring the temporal control of supramolecular events, has revealed the ability to produce an almost arbitrary distribution between substrates and complexes that is kinetically stable and difficult to obtain by other means. The breakthrough presented in this piece of work has opened up an innovative approach for manipulating noncovalent synthesis, pushing self-assembly towards a new level with precise, adaptive and life-like nature. Further reading: C&EN, Chemistry World, Northwestern Now, EurekAlert!, PHYS. ORG, XMOL, and DeepTech.
The Chem Highlight of this work—entitled “Electron Catalysis Expands the Supramolecular Chemist’s Toolbox” by Professor Rafal Klajn and Mr Julius Gemen in the Department of Organic Chemistry, Weizmann Institute of Science, Israel—notes that: “Stoddart and co-workers report that the formation of supramolecular host-guest complexes can also benefit from the advantages of electrocatalysis… Recently in Nature, they report that electrons can initiate radical chain reactions that effectively control the rate of molecular recognition… In the context of systems chemistry, the ability to catalyze molecular recognition processes and control the time scales of supramolecular self-assembly will facilitate the development of new chemical reaction networks.”
In addition, Rafal and Julius have pointed out some drawbacks of our current research, such as the limited turnover number (TON). They offer suggestions for addressing this issue, along the lines of “Let us imagine multiple copies of the thread confined on the surface of a gold nanoparticle, where the ring components move freely in the surrounding solution. A one-electron reduction of either component would trigger a recognition event, and the resulting host–guest complex would find itself in close proximity to free threads attached to the same nanoparticle, thus transferring the electron in a highly efficient fashion. The chain of electron transfer could, in principle, continue until all the nanoparticle-bound threads are complexed with the rings, thus significantly increasing the TON and the turnover frequency of the process.”
Congratulations, Yang and Yunyan! Thanks to Rafal for the highlight and thoughtful comments!
Pictured below — A group photo of Fraser, Yang and Yunyan, and the TOC of Electron Catalysis: