A Research Article entitled “Corralarenes: A Family of Conjugated Tubular Hosts” has been published recently in Journal of the American Chemical Society (JACS) by Postdoctoral Fellow Han Han, collaborating with Professor Kang Cai from Nankai University.
Macrocycles have been front and center ever since the beginning of what has become known as either host–guest or supramolecular chemistry. Despite the abundance of synthetic macrocycles, macrocyclic hosts with relatively deep cavities are highly attractive because of their enhanced host–guest binding interactions and nanoconfined environments which are desirable for various applications, such as transmembrane mass transport, molecular separation, and supramolecular catalysis.
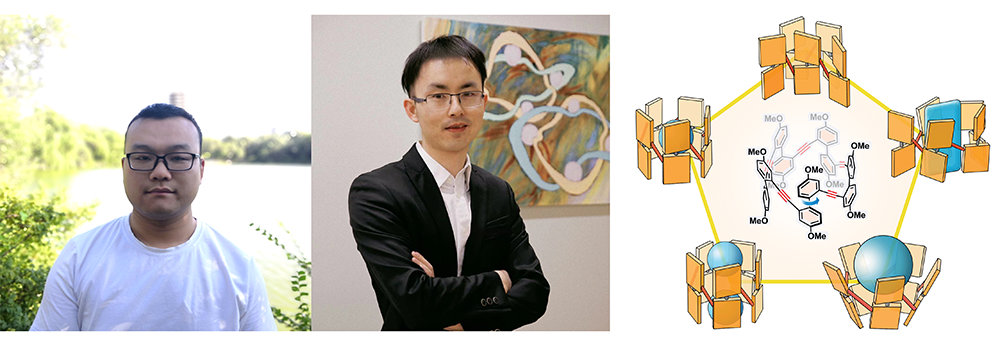
In this Article, the design and synthesis of two new tubular hosts—namely corral[4]arene and corral[5]arene, have been described. The former has been isolated and characterized as two conformational diastereoisomers: one is centrosymmetric and the other asymmetric. The latter, a fivefold symmetrical and flexible host, has also been investigated in considerable detail. It is composed of five 4,4′-dimethoxybiphenyl units bridged by ethynylene linkers at their 2,2′-positions and adopts a pentagonal conformation with a tubular-shaped cavity in the presence of guests. This structure endows corral[5]arene, not only with a conjugated backbone, capable of bright fluorescent emission, but also a deep π-electron-rich aromatic cavity with remarkable conformational flexibility. The adaptive cavity of corral[5]arene allows it to accommodate a wide range of neutral and positively charged electron-deficient guests with different molecular sizes and shapes. Binding constants between this host and these guests in three different nonpolar organic solvents lie in the range of 103 to 107 M–1. Moreover, corral[5]arene exhibits dynamic chirality on account of the axes of chirality associated with each of the five biphenyl units and displays first-order transformation as exhibited by circular dichroism in response to the addition of chiral guests. All these stereochemical features render corral[5]arene an attractive host for a variety of supramolecular and nanotechnological applications.
This Research Article has been selected as one of the covers by JACS. Congratulations, Han and Kang! A terrific piece of research and a great start by the Kang Cai Laboratory.
Pictured below – Han Han and Kang Cai alongside the TOC for their JACS publication “Corralarenes: A Family of Conjugated Tubular Hosts”: