The long-standing challenge of trapping an active native phlorin intermediate in the past six decades has been cracked! A communication entitled “Mechanical trapping of the phlorin intermediate” has been published in CCS Chem by former Postdoctoral Research Fellow Zhichang Liu.
Trapping unstable intermediates for elucidating reaction mechanisms in chemistry presents a formidable challenge. There has long been a lack of direct evidence for key intermediates involving, for example, the highly reactive phlorin leading to porphyrin.
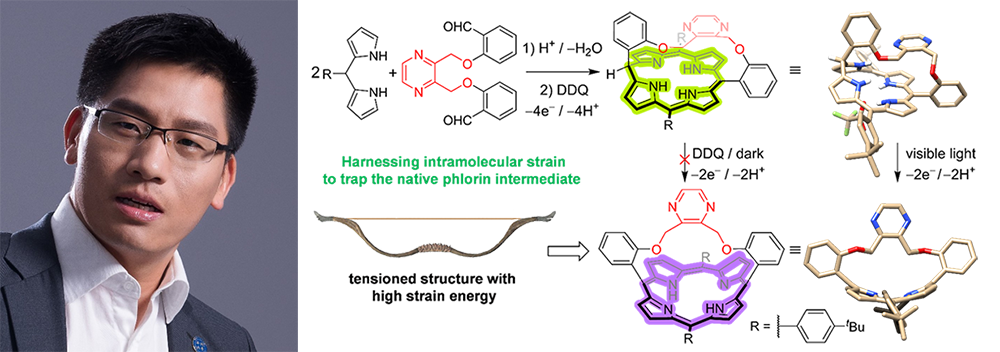
This communication describes the use of a molecular-strain engineering (MSE) strategy, involving the harnessing of intramolecular strain, to trap the native phlorin during porphyrin synthesis. By mechanically constraining its periphery, a phlorin, which is stable towards oxidation, has been captured as an isolable intermediate and fully characterized. This strained intermediate can be transformed quantitatively into the corresponding bow-shaped porphyrin upon photoirradiation. Theoretical calculations indicate that the constraint imposed on the phlorin enhances the activation energy to reach the transition state of its oxidative aromatization and destabilizes the resulting porphyrin, thus facilitating trapping of this highly reactive intermediate. This proof-of-concept demonstration establishes the potential of highly creative MSE to capture native intermediates using mechanical stress as a transformative alternative to adding scavengers and opens up a convenient way for carrying out in-depth mechanistic investigations.
Zhichang was a former Postdoctoral Fellow in the Group. He started his independent academic career in China as an Assistant Professor of Chemistry—the first faculty in chemistry—at Westlake University on September 1, 2018. His current research interests include molecular-strain engineering and supramolecular organic functional assemblies (SOFA).
Congratulations to Zhichang! A terrific job by his SOFA LAB.
Pictured below — Zhichang Liu alongside the TOC for his publication in CCS Chem: